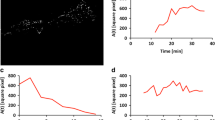
Abstract
The stiffness of adherent mammalian cells is regulated by the elasticity of substrates due to mechanotransduction via integrin-based focal adhesions. Dictyostelium discoideum is an ameboid protozoan model organism that does not carry genes for classical integrin and can adhere to substrates without forming focal adhesions. It also has a life cycle that naturally includes both single-cellular and multicellular life forms. In this article, we report the measurements of the elastic modulus of single cells on varied substrate stiffnesses and the elastic modulus of the multicellular “slug” using atomic force microscopy (AFM) as a microindenter/force transducer. The results show that the elastic modulus of the Dictyostelium cell is regulated by the stiffness of the substrate and its surrounding cells, which is similar to the mechanotransduction behavior of mammalian cells.





Similar content being viewed by others
References
Iskratsch, T., Wolfenson, H., Sheetz, M.P.: Appreciating force and shape — the rise of mechanotransduction in cell biology. Nat. Rev. Mol. Cell Biol. 15, 825–833 (2014). https://doi.org/10.1038/nrm3903
Discher, D.E.: Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 (2005). https://doi.org/10.1126/science.1116995
Kshitiz, Park, J., Kim, P., Helen, W., Engler, A.J., Levchenko, A., Kim, D.-H.: Control of stem cell fate and function by engineering physical microenvironments. Integr. Biol. (Camb) 4, 1008–1018 (2012)
Engler, A.J., Griffin, M.A., Sen, S., Bönnemann, C.G., Sweeney, H.L., Discher, D.E.: Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell Biol. 166, 877–887 (2004). https://doi.org/10.1083/jcb.200405004
Moeendarbary, E., Harris, A.R.: Cell mechanics: principles, practices, and prospects: cell mechanics. Wiley Interdiscip. Rev. Syst. Biol. Med. 6, 371–388 (2014). https://doi.org/10.1002/wsbm.1275
Solon, J., Levental, I., Sengupta, K., Georges, P.C., Janmey, P.A.: Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys. J. 93, 4453–4461 (2007). https://doi.org/10.1529/biophysj.106.101386
Byfield, F.J., Reen, R.K., Shentu, T.-P., Levitan, I., Gooch, K.J.: Endothelial actin and cell stiffness is modulated by substrate stiffness in 2D and 3D. J. Biomech. 42, 1114–1119 (2009). https://doi.org/10.1016/j.jbiomech.2009.02.012
Takai, E., Costa, K.D., Shaheen, A., Hung, C.T., Guo, X.E.: Osteoblast elastic modulus measured by atomic force microscopy is substrate dependent. Ann. Biomed. Eng. 33, 963–971 (2005). https://doi.org/10.1007/s10439-005-3555-3
Yousafzai, M.S., Ndoye, F., Coceano, G., Niemela, J., Bonin, S., Scoles, G., Cojoc, D.: Substrate-dependent cell elasticity measured by optical tweezers indentation. Opt. Lasers Eng. 76, 27–33 (2016). https://doi.org/10.1016/j.optlaseng.2015.02.008
Wang, N., Tytell, J.D., Ingber, D.E.: Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 10, 75–82 (2009). https://doi.org/10.1038/nrm2594
Loomis, W.F., Fuller, D., Gutierrez, E., Groisman, A., Rappel, W.-J.: Innate non-specific cell substratum adhesion. PLoS One 7, e42033 (2012). https://doi.org/10.1371/journal.pone.0042033
Cornillon, S., Gebbie, L., Benghezal, M., Nair, P., Keller, S., Wehrle-Haller, B., Charette, S.J., Brückert, F., Letourneur, F., Cosson, P.: An adhesion molecule in free-living Dictyostelium amoebae with integrin β features. EMBO Rep. (2006). https://doi.org/10.1038/sj.embor.7400701
Cornillon, S., Froquet, R., Cosson, P.: Involvement of Sib proteins in the regulation of cellular adhesion in Dictyostelium discoideum. Eukaryot. Cell 7, 1600–1605 (2008). https://doi.org/10.1128/EC.00155-08
Luo, T., Mohan, K., Iglesias, P.A., Robinson, D.N.: Molecular mechanisms of cellular mechanosensing. Nat. Mater. 12, 1064–1071 (2013). https://doi.org/10.1038/nmat3772
Orr, A.W., Helmke, B.P., Blackman, B.R., Schwartz, M.A.: Mechanisms of mechanotransduction. Dev. Cell 10, 11–20 (2006). https://doi.org/10.1016/j.devcel.2005.12.006
Chen, G., Zhuchenko, O., Kuspa, A.: Immune-like phagocyte activity in the social amoeba. Science 317, 678–681 (2007). https://doi.org/10.1126/science.1143991
Oyen, M.L.: Nanoindentation of biological and biomimetic materials. Exp. Tech. 37, 73–87 (2013). https://doi.org/10.1111/j.1747-1567.2011.00716.x
Ding, Y., Xu, G.-K., Wang, G.-F.: On the determination of elastic moduli of cells by AFM based indentation. Sci. Rep. 7, (2017). https://doi.org/10.1038/srep45575
Haupt, B.J., Osbourn, M., Spanhoff, R., de Keijzer, S., Müller-Taubenberger, A., Snaar-Jagalska, E., Schmidt, T.: Asymmetric elastic properties of Dictyostelium discoideum in relation to chemotaxis. Langmuir 23, 9352–9357 (2007). https://doi.org/10.1021/la700693f
Butt, H.-J., Cappella, B., Kappl, M.: Force measurements with the atomic force microscope: technique, interpretation and applications. Surf. Sci. Rep. 59, 1–152 (2005). https://doi.org/10.1016/j.surfrep.2005.08.003
Hutter, J.L., Bechhoefer, J.: Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 64, 1868–1873 (1993). https://doi.org/10.1063/1.1143970
Mahaffy, R.E., Shih, C.K., MacKintosh, F.C., Käs, J.: Scanning probe-based frequency-dependent microrheology of polymer gels and biological cells. Phys. Rev. Lett. 85, 880–883 (2000). https://doi.org/10.1103/PhysRevLett.85.880
Jacobs, T.D.B., Mathew Mate, C., Carpick, R.W.: Understanding the tip–sample contact: an overview of contact mechanics from the macro- to the nanoscale. In: Yablon, D.G. (ed.) Scanning Probe Microscopy in Industrial Applications, pp. 15–48. Wiley, Hoboken (2013)
Saha, R., Nix, W.D.: Effects of the substrate on the determination of thin film mechanical properties by nanoindentation. Acta Mater. 50, 23–38 (2002). https://doi.org/10.1016/S1359-6454(01)00328-7
Selby, A., Maldonado-Codina, C., Derby, B.: Influence of specimen thickness on the nanoindentation of hydrogels: measuring the mechanical properties of soft contact lenses. J. Mech. Behav. Biomed. Mater. 35, 144–156 (2014). https://doi.org/10.1016/j.jmbbm.2013.11.023
Kirmizis, D., Logothetidis, S.: Atomic force microscopy probing in the measurement of cell mechanics. Int. J. Nanomedicine 5, 137–145 (2010)
Normand, V., Lootens, D.L., Amici, E., Plucknett, K.P., Aymard, P.: New insight into agarose gel mechanical properties. Biomacromolecules. 1, 730–738 (2000). https://doi.org/10.1021/bm005583j
Stolz, M., Raiteri, R., Daniels, A.U., VanLandingham, M.R., Baschong, W., Aebi, U.: Dynamic elastic modulus of porcine articular cartilage determined at two different levels of tissue organization by indentation-type atomic force microscopy. Biophys. J. 86, 3269–3283 (2004). https://doi.org/10.1016/S0006-3495(04)74375-1
Ketene, A.N., Roberts, P.C., Shea, A.A., Schmelz, E.M., Agah, M.: Actin filaments play a primary role for structural integrity and viscoelastic response in cells. Integr. Biol. 4, 540 (2012). https://doi.org/10.1039/c2ib00168c
Discher, D.E., Smith, L., Cho, S., Colasurdo, M., García, A.J., Safran, S.: Matrix mechanosensing: from scaling concepts in ’omics data to mechanisms in the nucleus, regeneration, and cancer. Annu. Rev. Biophys. 46, 295–315 (2017). https://doi.org/10.1146/annurev-biophys-062215-011206
Coates, J.C., Harwood, A.J.: Cell-cell adhesion and signal transduction during Dictyostelium development. J. Cell Sci. 114, 4349–4358 (2001)
Farnsworth, P.A., Loomis, W.F.: A gradient in the thickness of the surface sheath in pseudoplasmodia of Dictyostelium discoideum. Dev. Biol. 46, 349–357 (1975). https://doi.org/10.1016/0012-1606(75)90111-6
Tasaka, M., Takeuchi, I.: Cell patterning during slug migration and early culmination in Dictyostelium discoideum. Differentiation. 23, 184–188 (1982). https://doi.org/10.1111/j.1432-0436.1982.tb01282.x
Acknowledgments
The authors acknowledge the support from Loras College (sabbatical awarded to K.M.C.) and the Iowa Science Foundation for this project (ISF 16-07 to K.M.C.). We thank Dr. Lee Farina in the Engineering Physics Department at University of Wisconsin-Platteville for her design of the microscope adaptor and her assistance in AFM experiments. The AFM experiments were carried out in the Material Fabrication and Nanoscale Characterization Lab under the support of the College of Engineering, Mathematics, and Science at University of Wisconsin-Platteville.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 323 kb)
Rights and permissions
About this article
Cite this article
Wu, Y., Cooper, K.M. Elastic modulus of Dictyostelium is affected by mechanotransduction. J Biol Phys 45, 293–305 (2019). https://doi.org/10.1007/s10867-019-09529-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10867-019-09529-1