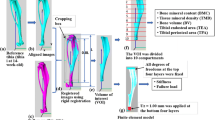
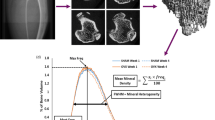
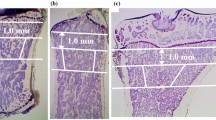
Abstract
It is well known that estrogen deficiency induces a deterioration of bone strength in aged females. The aim of this study is to determine the effect of estrogen depletion on tibia bone strength in sexually mature mice that are still undergoing skeletal maturation. At 8 weeks of age, C57BL/6 female mice underwent an ovariectomy (OVX) or sham (SHAM) surgery. Mice were killed at 2, 4, or 8 weeks post-surgery. Tibia length and cross-sectional area continued to increase in both treatment groups until 4 weeks post-surgery. Compared to SHAM mice, OVX mice demonstrated a significant reduction in uterine weight and plasma estrogen levels. Three-point bending was used to quantify the mechanical properties (breaking point, stress, stiffness, and elasticity) of the tibia. The tibias from the SHAM mice had a higher breaking point than all the age-matched OVX mice. At 8 weeks post-surgery, the tibias from the SHAM mice demonstrated higher elasticity, stress, and stiffness than the younger SHAM mice and the age-matched OVX mice. Compared to the SHAM mice, our study suggests that (1) there is a reduction in the mechanical strength of tibias from young OVX mice, and (2) the greatest decline in tibia strength of the OVX mice was once they reached skeletal maturity.



Similar content being viewed by others
References
Adeel, S., Singh, K., Vydareny, K.H., Kumari, M., Shah, E., Weitzmann, M.N., Tangpricha, V.: Bone loss in surgically ovariectomized pre-menopausal women is associated with T lymphocyte activation and thymic hypertrophy. J. Investig. Med.: Off. Publ. Am Fed. Clin. Res. 61(8), 1178–1183 (2013). doi:10.231/JIM.0000000000000016
Baykan, E.K., Erdoğan, M., Özen, S., Darcan, Ş., Saygılı, L.F.: Aromatase deficiency, a rare syndrome: case report. J. Clin. Res. Pediatr. Endocrinol. 5(2), 129–132 (2013). doi:10.4274/Jcrpe.970
Bulun, S.E.: Aromatase and estrogen receptor alpha deficiency. Fertil. Steril. 101(2), 323–329 (2014). doi:10.1016/j.fertnstert.2013.12.022
Almeida, O.M., Jorgetti, W., Oksman, D., Jorgetti, C., Rocha, D.L., Gemperli, R.: Comparative study and histomorphometric analysis of bone allografts lyophilized and sterilized by autoclaving, gamma irradiation and ethylene oxide in rats. Acta Cir. Bras. 28(1), 66–71 (2013)
Riggs, B.L.: Endocrine causes of age-related bone loss and osteoporosis. Novartis Found. Symp. 242, 247–259 (2002). discussion 260–244
Ornoy, A., Giron, S., Aner, R., Goldstein, M., Boyan, B.D., Schwartz, Z.: Gender-dependent effects of testosterone and 17 beta-estradiol on bone growth and modelling in young mice. Bone Miner. 24(1), 43–58 (1994)
Main, R.P., Lynch, M.E., van der Meulen, M.C.: Load-induced changes in bone stiffness and cancellous and cortical bone mass following tibial compression diminish with age in female mice. J. Exp. Biol. 217(Pt 10), 1775–1783 (2014). doi:10.1242/jeb.085522
Lang, D.H., Sharkey, N.A., Lionikas, A., Mack, H.A., Larsson, L., Vogler, G.P., Vandenbergh, D.J., Blizard, D.A., Stout, J.T., Stitt, J.P., McClearn, G.E.: Adjusting data to body size: a comparison of methods as applied to quantitative trait loci analysis of musculoskeletal phenotypes. J. Bone Miner. Res. 20(5), 748–757 (2005). doi:10.1359/JBMR.041224
Razi, H., Birkhold, A.I., Zaslansky, P., Weinkamer, R., Duda, G.N., Willie, B.M., Checa, S.: Skeletal maturity leads to a reduction in the strain magnitudes induced within the bone: a murine tibia study. Acta Biomater. 13, 301–310 (2015). doi:10.1016/j.actbio.2014.11.021
Bouxsein, M.L., Myers, K.S., Shultz, K.L., Donahue, L.R., Rosen, C.J., Beamer, W.G.: Ovariectomy-induced bone loss varies among inbred strains of mice. J. Bone Miner. Res. 20(7), 1085–1092 (2005). doi:10.1359/JBMR.050307
Nelson, J.F., Karelus, K., Felicio, L.S., Johnson, T.E.: Genetic influences on the timing of puberty in mice. Biol. Reprod. 42(4), 649–655 (1990)
Glatt, V., Canalis, E., Stadmeyer, L., Bouxsein, M.L.: Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J. Bone Miner. Res. 22(8), 1197–1207 (2007). doi:10.1359/jbmr.070507
Somerville, J.M., Aspden, R.M., Armour, K.E., Armour, K.J., Reid, D.M.: Growth of C57BL/6 mice and the material and mechanical properties of cortical bone from the tibia. Calcif. Tissue Int. 74(5), 469–475 (2004). doi:10.1007/s00223-003-0101-x
Beamer, W.G., Donahue, L.R., Rosen, C.J., Baylink, D.J.: Genetic variability in adult bone density among inbred strains of mice. Bone 18(5), 397–403 (1996)
Cao, J.J., Gregoire, B.R.: A high-fat diet increases body weight and circulating estradiol concentrations but does not improve bone structural properties in ovariectomized mice. Nutr. Res. 36(4), 320–327 (2016). doi:10.1016/j.nutres.2015.12.008
Walker, A.H., Perkins, O., Mehta, R., Ali, N., Dobretsov, M., Chowdhury, P.: Changes in mechanical properties of rat bones under simulated effects of microgravity and radiation. Phys. Procedia 66, 610–616 (2015). doi:10.1016/j.phpro.2015.05.081
Jiao, F., Chiu, H., Jiao, Y., de Rijk, W.G., Li, X., Eckstein, E.C., Beamer, W.G., Gu, W.: Quantitative trait loci for tibial bone strength in C57BL/6J and C3H/HeJ inbred strains of mice. J. Genet. 89(1), 21–27 (2010)
Tuukkanen, J., Koivukangas, A., Jamsa, T., Sundquist, K., Mackay, C.A., Marks Jr., S.C.: Mineral density and bone strength are dissociated in long bones of rat osteopetrotic mutations. J. Bone Miner. Res. 15(10), 1905–1911 (2000). doi:10.1359/jbmr.2000.15.10.1905
Ritchie, R.O., Koester, K.J., Ionova, S., Yao, W., Lane, N.E., Ager 3rd, J.W.: Measurement of the toughness of bone: a tutorial with special reference to small animal studies. Bone 43(5), 798–812 (2008). doi:10.1016/j.bone.2008.04.027
Kamal, B., Russell, D., Payne, A., Constante, D., Tanner, K.E., Isaksson, H., Mathavan, N., Cobb, S.R.: Biomechanical properties of bone in a mouse model of Rett syndrome. Bone 71, 106–114 (2015). doi:10.1016/j.bone.2014.10.008
Windahl, S.H., Andersson, N., Chagin, A.S., Martensson, U.E., Carlsten, H., Olde, B., Swanson, C., Moverare-Skrtic, S., Savendahl, L., Lagerquist, M.K., Leeb-Lundberg, L.M., Ohlsson, C.: The role of the G protein-coupled receptor GPR30 in the effects of estrogen in ovariectomized mice. Am. J. Physiol. Endocrinol. Metab. 296(3), E490–496 (2009). doi:10.1152/ajpendo.90691.2008
Stubbins, R.E., Holcomb, V.B., Hong, J., Nunez, N.P.: Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur. J. Nutr. 51(7), 861–870 (2012). doi:10.1007/s00394-011-0266-4
Iwaniec, U.T., Turner, R.T.: Influence of body weight on bone mass, architecture and turnover. J. Endocrinol. 230(3), R115–130 (2016). doi:10.1530/JOE-16-0089
Rouach, V., Katzburg, S., Koch, Y., Stern, N., Somjen, D.: Bone loss in ovariectomized rats: dominant role for estrogen but apparently not for FSH. J. Cell. Biochem. 112(1), 128–137 (2011). doi:10.1002/jcb.22908
Sun, L., Peng, Y., Sharrow, A.C., Iqbal, J., Zhang, Z., Papachristou, D.J., Zaidi, S., Zhu, L.L., Yaroslavskiy, B.B., Zhou, H., Zallone, A., Sairam, M.R., Kumar, T.R., Bo, W., Braun, J., Cardoso-Landa, L., Schaffler, M.B., Moonga, B.S., Blair, H.C., Zaidi, M.: FSH directly regulates bone mass. Cell 125(2), 247–260 (2006). doi:10.1016/j.cell.2006.01.051
Ozbek, M.N., Demirbilek, H., Baran, R.T., Baran, A.: Bone mineral density in adolescent girls with hypogonadotropic and hypergonadotropic hypogonadism. J. Clin. Res. Pediatr. Endocrinol. 8(2), 163–169 (2016). doi:10.4274/jcrpe.2228
Nakamura, T., Imai, Y., Matsumoto, T., Sato, S., Takeuchi, K., Igarashi, K., Harada, Y., Azuma, Y., Krust, A., Yamamoto, Y., Nishina, H., Takeda, S., Takayanagi, H., Metzger, D., Kanno, J., Takaoka, K., Martin, T.J., Chambon, P., Kato, S.: Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 130(5), 811–823 (2007). doi:10.1016/j.cell.2007.07.025
Wang, L., Liu, S., Zhao, Y., Liu, D., Liu, Y., Chen, C., Karray, S., Shi, S., Jin, Y.: Osteoblast-induced osteoclast apoptosis by fas ligand/FAS pathway is required for maintenance of bone mass. Cell Death Differ. 22(10), 1654–1664 (2015). doi:10.1038/cdd.2015.14
Moverare-Skrtic, S., Wu, J., Henning, P., Gustafsson, K.L., Sjogren, K., Windahl, S.H., Koskela, A., Tuukkanen, J., Borjesson, A.E., Lagerquist, M.K., Lerner, U.H., Zhang, F.P., Gustafsson, J.A., Poutanen, M., Ohlsson, C.: The bone-sparing effects of estrogen and WNT16 are independent of each other. Proc. Natl. Acad. Sci. U. S. A. 112(48), 14972–14977 (2015). doi:10.1073/pnas.1520408112
Todd, H., Galea, G.L., Meakin, L.B., Delisser, P.J., Lanyon, L.E., Windahl, S.H., Price, J.S.: Wnt16 is associated with age-related bone loss and estrogen withdrawal in murine bone. PLoS ONE 10(10), e0140260 (2015). doi:10.1371/journal.pone.0140260
LeBlanc, A.J., Reyes, R., Kang, L.S., Dailey, R.A., Stallone, J.N., Moningka, N.C., Muller-Delp, J.M.: Estrogen replacement restores flow-induced vasodilation in coronary arterioles of aged and ovariectomized rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297(6), R1713–1723 (2009). doi:10.1152/ajpregu.00178.2009
Acknowledgments
Support was provided by grants from the National Institutes of Health (NIH) National Institute of General Medical Sciences (NIGMS) (P20 GM103429) to B.J.F.H. and the Arkansas Space Grant Consortium to A.W. Assistance was provided by Dr. Rahul Mehta (Dept of Physics & Astronomy) and Otis Perkins (undergraduate student). Analysis of plasma estrogen was conducted by The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core, which is supported by the Eunice Kennedy Shriver NICHD/NIH (SCCPIR) Grant U54-HD28934.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deckard, C., Walker, A. & Hill, B.J.F. Using three-point bending to evaluate tibia bone strength in ovariectomized young mice. J Biol Phys 43, 139–148 (2017). https://doi.org/10.1007/s10867-016-9439-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10867-016-9439-y