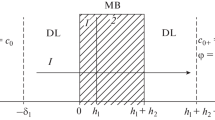
Abstract
Kinetics of facilitated ion transport through planar bilayer membranes are normally analyzed by electrical conductance methods. The additional use of electrical relaxation techniques, such as voltage jump, is necessary to evaluate individual rate constants. Although electrochemical impedance spectroscopy is recognized as the most powerful of the available electric relaxation techniques, it has rarely been used in connection with these kinetic studies. According to the new approach presented in this work, three steps were followed. First, a kinetic model was proposed that has the distinct quality of being general, i.e., it properly describes both carrier and channel mechanisms of ion transport. Second, the state equations for steady-state and for impedance experiments were derived, exhibiting the input–output representation pertaining to the model’s structure. With the application of a method based on the similarity transformation approach, it was possible to check that the proposed mechanism is distinguishable, i.e., no other model with a different structure exhibits the same input–output behavior for any input as the original. Additionally, the method allowed us to check whether the proposed model is globally identifiable (i.e., whether there is a single set of fit parameters for the model) when analyzed in terms of its impedance response. Thus, our model does not represent a theoretical interpretation of the experimental impedance but rather constitutes the prerequisite to select this type of experiment in order to obtain optimal kinetic identification of the system. Finally, impedance measurements were performed and the results were fitted to the proposed theoretical model in order to obtain the kinetic parameters of the system. The successful application of this approach is exemplified with results obtained for valinomycin–K+ in lipid bilayers supported onto gold substrates, i.e., an arrangement capable of emulating biological membranes.
Similar content being viewed by others
References
Terrettaz, S., Stora, T., Duschl, C., Vogel, H.: Protein binding to supported lipid membranes: investigation of the cholera toxin–ganglioside interaction by simultaneous impedance spectroscopy and surface plasmon resonance. Langmuir 9, 1361–1369 (1993)
Peggion, C., Formaggio, F., Toniolo, C., Becucci, L., Moncelli, M.R., Guidelli, R.: A peptide-tethered lipid bilayer on mercury as a biomimetic system. Langmuir 17, 6585–6592 (2001)
Bakker, E., Meyerhoff, M.E.: Ionophore-based membrane electrodes: new analytical concepts and non-classical response mechanisms. Anal. Chim. Acta 416, 121–137 (2000)
Starzak, M.: Resolving linear and nonlinear interactive kinetic mechanisms for ions in membrane channels. J. Biol. Phys. 23, 133–142 (1997)
Shirai, O., Yamana, J., Ohnuki, T., Yoshida, Y., Kihara, S.: Ion transport across a bilayer lipid membrane facilitated by valinomycin. J. Electroanal. Chem. 570, 219–226 (2004)
Forester, T.R., Smith, W., Clarke, J.H.R.: Capture of potassium ions by valinomycin: a molecular dynamics simulation study. J. Phys. Chem. 99, 14418–14423 (1995)
Pullman, A.: Contribution of theoretical chemistry to the study of ion transport through membranes. Chem. Rev. 91, 793–812 (1991)
Stark, G., Ketterer, B., Benz, R., Läuger, P.: The rate constant of valinomycin-mediated ion transport through thin lipid membranes. Biophys. J. 11, 981–993 (1971)
Horn, L.W.: A novel method for the observation of membrane transporter dynamics. Biophys. J. 64, 281–289 (1993)
Berthier, F., Diard, J.P., Pronzato, L., Walter, E.: Identifiability and distinguishability concepts in electrochemistry. Automatica 32, 973–984 (1996)
Stelzle, M., Weissmüller, G., Sackmann, E.: On the application of supported bilayers as receptive layers for biosensors with electrical detection. J. Phys. Chem. 97, 2974–2981 (1993)
Nollert, P., Kiefer, H., Jähnig, F.: Lipid vesicle adsorption versus formation of planar bilayers on solid surfaces. Biophys. J. 69, 1447–1455 (1995)
de Levie, R.: Mathematical modeling of transport of lipid-soluble ions and ion-carrier complexes through lipid bilayer membranes. Adv. Chem. Phys. 37, 99–137 (1978)
Gervasi, C.A., Vallejo, A.E.: Sodium ion transport through gramicidin-doped bilayers. influences of temperature and ionic concentration. Electrochim. Acta 47, 2259–2264 (2002)
Vallejo, A.E., Gervasi, C.A.: Impedance analysis of ion transport through gramicidin channels in supported lipid bilayers. Bioelectrochemistry 57, 1–7 (2002)
Senda, M., Kakiuchi, T., Osakai, T.: Electrochemistry at the interface between two immiscible electrolyte solutions. Electrochim. Acta 36, 253–262 (1991)
Banysek, P., Ruth, W., Koryta, J.: Valinomycin mediated transfer of potassium across the water/nitrobenzene interface. A study by voltammetry at the interface between two immiscible electrolyte solutions. J. Electroanal. Chem. 148, 117–121 (1983)
Forester, T.R., Smith, W., Clarke, J.H.R.: Antibiotic activity of valinomycin molecular dynamics simulations involving the water/membrane interface. J. Chem. Soc. Faraday Trans. 93, 613–619 (1997)
Naumann, R., Walz, D., Schiller, S.M., Knoll, W.: Kinetics of valinomycin-mediated K+ ion transport through tethered bilayer lipid membranes. J. Electroanal. Chem. 550–551, 241–252 (2003)
Kakutani, T., Nishiwaki, Y., Osakai, T., Senda, M.: On the mechanism of transfer of sodium ion across the nitrobenzene/water interface facilitated by dibenzo-18-crown-6. Bull. Chem. Soc. Jpn. 59, 781–788 (1986)
Berthier, F., Diard, J.P., Landaud, P., Montella, C.: Steady-state investigation and electrochemical impedance spectroscopy: identifiability and distinguishability of metal dissolution and passivation mechanisms. J. Electroanal. Chem. 362, 13–20 (1993)
Gabrielli, C.: Identification of Electrochemical Processes by Frequency Response Analysis. Technical Report No. 004. Solartron, Hampshire (1984)
Vallejo, A.E., Gervasi, C.A.: On the use of impedance spectroscopy for studying bilayer lipid membranes. In: Tien, H.T., Ottova, A. (eds.) Advances in Planar Lipid Bilayers and Liposomes, vol 3, pp. 331–353. Elsevier, Amsterdam (2006)
Milocco, R.H., Castro, E.B., Real, S.G.: Parameter identification and model validation of adsorption and electron transfer processes using impedance. Electrochim. Acta 47, 2035–2041 (2002)
Milocco, R.H.: Structural identifiability of adsorption and electron transfer processes in metal dissolution and passivation mechanisms. J. Electroanal. Chem. 471, 1–8 (1999)
Walter, E., Pronzato, L.: Identification of Parameter Models from Experimental Data. Springer, Berlin (1997)
Vallejo, A.E., Gervasi, C.A.: EIS studies of valinomycin-mediated K+ transport through supported lipid bilayers. J. Electroanal. Chem. 603, 51–58 (2007)
Benz, R., Stark, G., Jandko, K., Läuger, P.: Valinomycin-mediated ion transport through neutral lipid membranes: influence of hydrocarbon chain length and temperature. J. Membr. Biol. 14, 339–364 (1973)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alvarez, P.E., Gervasi, C.A. & Vallejo, A.E. Impedance Analysis of Ion Transport Through Supported Lipid Membranes Doped with Ionophores: A New Kinetic Approach. J Biol Phys 33, 421–431 (2007). https://doi.org/10.1007/s10867-008-9072-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10867-008-9072-5