Abstract
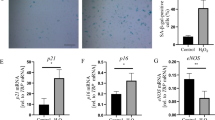
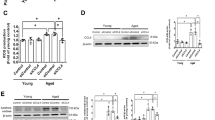
Endothelial dysfunction is a key early link in the pathogenesis of atherosclerosis, and the accumulation of senescent vascular endothelial cells causes endothelial dysfunction. Phosphoenolpyruvate (PEP), which is a high-energy glycolytic intermediate, protects against ischemia-reperfusion injury in isolated rat lung, heart, and liver tissue by quickly providing ATP. However, it was reported that serum PEP concentrations are 13-fold higher in healthy elderly compare to the young. Unlike that of other cell types, the energy required for the physiological function of endothelial cells is mainly derived from glycolysis. Recently, it is unclear whether circulating accumulation of PEP affects endothelial cell function. In this study, we found for the first time that 50-250 μM of PEP significantly promoted THP-1 monocyte adhesion to human umbilical vein endothelial cells (HUVECs) through increased expression of vascular endothelial adhesion factor 1 (VCAM1) and intercellular adhesion factor 1 (ICAM1) in HUVECs. Meanwhile, 50-250 μM of PEP decreased the expression of endothelial nitric oxide synthase (eNOS) and cellular level of nitric oxide (NO) in HUVECs. Moreover, PEP increased levels of ROS, enhanced the numbers of SA-β-Gal-positive cells and upregulated the expression of cell cycle inhibitors such as p21, p16 and the phosphorylation level of p53 on Ser15, and the expression of proinflammatory factors including TNF-α, IL-1β, IL-6, IL-8, IL-18 and MCP-1 in HUVECs. Furthermore, PEP increased both oxygen consumption rate (OCR) and glycolysis rate, and was accompanied by reduced NAD+/NADH ratios and enhanced phosphorylation levels of AMPKα (Thr172), p38 MAPK (T180/Y182) and NF-κB p65 (Ser536) in HUVECs. Notably, PEP had no significant effect on hepG2 cells. In conclusion, these results demonstrated that PEP induced dysfunction and senescence in vascular endothelial cells through stimulation of metabolic reprogramming.




Similar content being viewed by others
Data availability statement
The data that support the findings of this study are available from the corresponding author, [WT], upon reasonable request.
References
Abulizi A, Cardone RL, Stark R, Lewandowski SL, Zhao X, Hillion J, Ma L, Sehgal R, Alves TC, Thomas C, Kung C, Wang B, Siebel S, Andrews ZB, Mason GF, Rinehart J, Merrins MJ, Kibbey RG (2020) Multi-Tissue Acceleration of the Mitochondrial Phosphoenolpyruvate Cycle Improves Whole-Body Metabolic Health. Cell Metab 32:751-766.e711. https://doi.org/10.1016/j.cmet.2020.10.006
Andrew PJ, Mayer B (1999) Enzymatic function of nitric oxide synthases. Cardiovasc Res 43:521–531. https://doi.org/10.1016/s0008-6363(99)00115-7
Birch J, Gil J (2020) Senescence and the SASP: many therapeutic avenues. Genes Dev 34:1565–1576. https://doi.org/10.1101/gad.343129.120
Chang E, Harley CB (1995) Telomere length and replicative aging in human vascular tissues. Proc Natl Acad Sci USA 92:11190–11194. https://doi.org/10.1073/pnas.92.24.11190
Cuollo L, Antonangeli F, Santoni A, Soriani A (2020) The senescence-associated secretory phenotype (SASP) in the challenging future of cancer therapy and age-related diseases. Biology (Basel) 9(12):485. https://doi.org/10.3390/biology9120485
Félétou M (2011) Integrated Systems Physiology: from Molecule to Function to Disease, The Endothelium: Part 1: Multiple Functions of the Endothelial Cells—Focus on Endothelium-Derived Vasoactive Mediators, Morgan & Claypool Life Sciences Copyright © 2011 by Morgan & Claypool Life Sciences Publishers., San Rafael (CA)
Förstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33(829–837):837a–837d. https://doi.org/10.1093/eurheartj/ehr304
Gao M, Fu J, Wang Y (2020) The lncRNA FAL1 protects against hypoxia-reoxygenation- induced brain endothelial damages through regulating PAK1. J Bioenerg Biomembr 52:17–25. https://doi.org/10.1007/s10863-019-09819-2
Geng X, Shen J, Li F, Yip J, Guan L, Rajah G, Peng C, DeGracia D, Ding Y (2021) Phosphoenolpyruvate Carboxykinase (PCK) in the Brain Gluconeogenic Pathway Contributes to Oxidative and Lactic Injury After Stroke. Mol Neurobiol 58:2309–2321. https://doi.org/10.1007/s12035-020-02251-3
Gimbrone MA Jr, García-Cardeña G (2016) Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ Res 118:620–636. https://doi.org/10.1161/circresaha.115.306301
Gomes AP, Ilter D, Low V, Endress JE, Fernández-García J, Rosenzweig A, Schild T, Broekaert D, Ahmed A, Planque M, Elia I, Han J, Kinzig C, Mullarky E, Mutvei AP, Asara J, de Cabo R, Cantley LC, Dephoure N, Fendt SM, Blenis J (2020) Age-induced accumulation of methylmalonic acid promotes tumour progression. Nature 585:283–287. https://doi.org/10.1038/s41586-020-2630-0
Grasmann G, Smolle E, Olschewski H, Leithner K (1872) Gluconeogenesis in cancer cells - Repurposing of a starvation-induced metabolic pathway?, Biochimica et biophysica acta. Rev Cancer 2019:24–36. https://doi.org/10.1016/j.bbcan.2019.05.006
Libby P (2021) The changing landscape of atherosclerosis. Nature 592:524–533. https://doi.org/10.1038/s41586-021-03392-8
Luengo A, Li Z, Gui DY, Sullivan LB, Zagorulya M, Do BT, Ferreira R, Naamati A, Ali A, Lewis CA, Thomas CJ, Spranger S, Matheson NJ, Vander Heiden MG (2021) Increased demand for NAD(+) relative to ATP drives aerobic glycolysis. Mol Cell 81:691-707.e696. https://doi.org/10.1016/j.molcel.2020.12.012
Matacchione G, Gurău F, Silvestrini A, Tiboni M, Mancini L, Valli D, Rippo MR, Recchioni R, Marcheselli F, Carnevali O, Procopio AD, Casettari L, Olivieri F (2021) Anti-SASP and anti-inflammatory activity of resveratrol, curcumin and β-caryophyllene association on human endothelial and monocytic cells. Biogerontology 22:297–313. https://doi.org/10.1007/s10522-021-09915-0
Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I (2002) Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 105:1541–1544. https://doi.org/10.1161/01.cir.0000013836.85741.17
Nacarelli T, Lau L, Fukumoto T, Zundell J, Fatkhutdinov N, Wu S, Aird KM, Iwasaki O, Kossenkov AV, Schultz D, Noma KI, Baur JA, Schug Z, Tang HY, Speicher DW, David G, Zhang R (2019) NAD(+) metabolism governs the proinflammatory senescence-associated secretome. Nat Cell Biol 21:397–407. https://doi.org/10.1038/s41556-019-0287-4
Okuda K, Khan MY, Skurnick J, Kimura M, Aviv H, Aviv A (2000) Telomere attrition of the human abdominal aorta: relationships with age and atherosclerosis. Atherosclerosis 152:391–398. https://doi.org/10.1016/s0021-9150(99)00482-7
Oshima Y, Okazaki N (2005) Augmentation of hypoxic pulmonary vasoconstriction in isolated rabbit lungs with phosphoenolpyruvate Masui. Japanese J Anesthesiol 54:1234–1240
Oshima Y, Minami Y, Sakamoto S, Yamasaki K, Mochida S, Funaki K, Moriyama N, Otsuki A, Inagaki Y (2015) Phosphoenolpyruvate administration protects ischemia-reperfusion injury in isolated rabbit lungs. J Anesth 29:635–638. https://doi.org/10.1007/s00540-014-1972-x
Saiki S, Yamaguchi K, Chijiiwa K, Shimizu S, Hamasaki N, Tanaka M (1997) Phosphoenolpyruvate prevents the decline in hepatic ATP and energy charge after ischemia and reperfusion injury in rats. J Surg Res 73:59–65. https://doi.org/10.1006/jsre.1997.5177
Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM (2000) Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 20:1262–1275. https://doi.org/10.1161/01.atv.20.5.1262
Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, Freund A, Shirakawa K, Lim HW, Davis SS, Ramanathan A, Gerencser AA, Verdin E, Campisi J (2016) Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab 23:303–314. https://doi.org/10.1016/j.cmet.2015.11.011
Ying W (2008) NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal 10:179–206. https://doi.org/10.1089/ars.2007.1672
Zhao W, Ma G, Chen X (2014) Lipopolysaccharide induced LOX-1 expression via TLR4/MyD88/ROS activated p38MAPK-NF-κB pathway. Vascul Pharmacol 63:162–172. https://doi.org/10.1016/j.vph.2014.06.008
Zhou C, Shang W, Yin SK, Shi H, Ying W (2021) Malate-Aspartate Shuttle Plays an Important Role in LPS-Induced Neuroinflammation of Mice Due to its Effect on STAT3 Phosphorylation. Front Mol Biosci 8:655687. https://doi.org/10.3389/fmolb.2021.655687
Funding
This work was supported by the Beijing Natural Science Foundation (7212086, 7232141), the National Key R&D Program of China (2021YFE0114200) and the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (grant no. 2021-I2M-1–050).
Author information
Authors and Affiliations
Contributions
Conceptualization, Weiqing Tang and Jian Li; Data curation, Tong An and Weiqing Tang; Formal analysis, Tong An and Weiqing Tang; Funding acquisition, Weiqing Tang and Jian Li; Investigation, Weiqing Tang; Methodology, Tong An, Xiaoyi Zhang, Xin Gao, Xiyue Zhang, Tao Shen, Hongxia Li, Lin Dou, Xiuqing Huang, Yong Man, Guoping Li and Weiqing Tang; Project administration, Weiqing Tang and Jian Li; Resources, Weiqing Tang and Jian Li; Software, Tong An, Xiaoyi Zhang and Weiqing Tang; Supervision, Weiqing Tang and Jian Li; Validation, Tong An; Visualization, Tong An and Weiqing Tang; Writing – original draft, Tong An; Writing – review & editing, Weiqing Tang and Jian Li.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflicts of interest
The authors declare no competing financial interests or personal relation-ships which have, or could be perceived to have, influenced the work reported in this article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
An, T., Zhang, X., Gao, X. et al. Phosphoenolpyruvate induces endothelial dysfunction and cell senescence through stimulation of metabolic reprogramming. J Bioenerg Biomembr 55, 103–114 (2023). https://doi.org/10.1007/s10863-023-09965-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-023-09965-8