Abstract
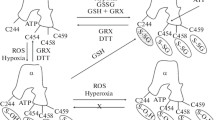
Na,K-ATPase is a member of the P-type ATPase family, which transforms the energy of ATP to the transmembrane Na/K gradient that is used to create membrane potential, support the excitability of neurons and myocytes, control pH, and transport substances. The regulation of the Na,K-ATPase function by physiological regulators also comprises a central role in the adaptation of organisms to different conditions. H2O2 is one of the main signaling molecules in redox metabolism and plays important function in cellular physiology. H2O2 also regulates signaling pathways via the specific oxidation of proteins harboring redox-sensitive moieties, like metal centers or cysteine residues, which control their activity. The Na,K-ATPase is redox-sensitive with an “optimal redox potential range,” where the reactive oxygen species (ROS), levels beyond this “optimal range” are responsible for enzyme inhibition. Thus reactive oxygen species manifest a hermetic effect, which is expressed by biphasic action; stimulation by low doses and inhibition by high doses. This study was aimed to reveal redox-sensitivity of brain synaptic membrane fractions Na,K-ATPase via H2O2 effects. Different concentrations of H2O2 change the kinetic parameters of the enzyme system for MgATP complex, Na+, and K+ differently. Moreover, H2O2 changes p-chloromercuribenzoic acids (PCMB) affinity. H2O2 targets thiols of the Na,K-ATPase – low and high concentrations of H2O2 change the oxidative state of thiolate (S‐) from Cys differently, resulting in the corresponding activation or inhibition of the enzyme. Targeting thiols of the Na,K-ATPase tunes the activity of the Na,K-ATPase to the cellular demands and sustains the enzyme activity at the “optimal” level.






Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Agekyan TA (1969) Fundamentals of the theory of errors for Astronomers and Physicists. Sov Astron 13:171
Bibert S, Liu CC, Figtree GA, Garcia A, Hamilton EJ, Marassi FM et al (2011) FXYD proteins reverse inhibition of the Na+-K+ pump mediated by glutathionylation of its beta1 subunit. J Biol Chem 286:18562–18572. https://doi.org/10.1074/jbc.M110.18410
Blanco G, Mercer RW (1998) Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol 275:F633–F650. https://doi.org/10.1152/ajprenal.1998.275.5.F633
Blanco G (2005) Na, K-ATPase subunit heterogeneity as a mechanism for tissue-specific ion regulation. Semin Nephrol 25:292–330. https://doi.org/10.1016/j.semnephrol.2005.03.004
Bogdanova A, Petrushanko I, Boldyrev A, Gassmann M (2006) Oxygen- and Redox-Induced Regulation of the Na/K ATPase. Curr Enzyme Inhibit 2:37–59. https://doi.org/10.2174/157340806775473490
Bublitz C, Lawler CA (1989) The activation of glucose dehydrogenase by p-chloromercuribenzoate. Mol Cell Biochem 86:101–106. https://doi.org/10.1007/BF00222609
Chkadua G, Nozadze E, Tsakadze L, Shioshvili L, Leladze M, Arutinova N, Dzneladze S, Javakhishvili M, Kupradze S (2022) Some Kinetic Features of Na, K-ATPase and Sensitivity to Noradrenalione. Cell Biochem Biophys 80(1):23–29. https://doi.org/10.1007/s12013-021-01032-6
Cremers CM, Jakob U (2013) Oxidant sensing by reversible disulfide bond formation. Biol Chem 288(37):26489–26496. https://doi.org/10.1074/jbc.R113.462929
De Robertis E (1969) Structural components of the synaptic region. Struct Neurochem 2:365–380. https://doi.org/10.1007/978-1-4615-7157-5_15
Finkel T (2011) Signal transduction by reactive oxygen species. J Cell Biol 194:7–15. https://doi.org/10.1083/jcb.201102095
Fiske GH, Subbarow Y (1925) The colorimetric determination of phosphorus. Biol Chem 66:375–400. https://doi.org/10.1016/S0021-9258(18)84756-1
Geering K (2006) FXYD proteins: new regulators of Na-K-ATPase. Am J Physiol Renal Physiol 290:F241–F250. https://doi.org/10.1152/ajprenal.00126.2005
Hernandez J (1992) Na, K-ATPase regulation by neurotransmitters. Neurochemistry Int 20(1):1–10. https://doi.org/10.1016/0197-0186(92)90119-c
Kazanov A, Maslova M (1984) The investigation of activation of Na, K-ATPase in the red blood cells of mammals. J Evol Biochem Physiol 16(5):81–87
Kometiani Z (2007) Kinetic analysis of multi-sited enzyme systems. Pub. House Sakartvelosmatsne, Tbilisi
Li Z, Neufeld GJ (2001) Isolation and characterization of mitochondrial F(1)-ATPase from crayfish (Orconectesvirilis) gills. Comp BiochemPhysiol B BiochemMol Biol 128:325–338. https://doi.org/10.1016/s1096-4959(00)00330-4
Liu J, Kennedy DJ, Yan Y, Shapiro JI (2012) Reactive Oxygen Species Modulation of Na/K-ATPase Regulates Fibrosis and Renal Proximal Tubular Sodium Handling. International Journal of Nephrology 3:ID381320. https://doi.org/10.1155/2012/381320
Lowry O, Rosenbrough N, Randall R (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193(1):265–275. https://doi.org/10.1016/S0021-9258(19)52451-6
Mao L, Franke J (2013) Hormesis in Aging and Neurodegeneration—A Prodigy Awaiting Dissection. nt. J Mol Sci 14:13109–13128. https://doi.org/10.3390/ijms140713109
Penefsky HS, Cross RL (1991) Structure and mechanism of FoF1-type ATP synthases and ATPases. Adv Enzymol Relat Areas Mol Biol 64:173–214. https://doi.org/10.1002/9780470123102.ch4
Rhee SG, Kang SW, Jeong W, Chang TS, Yang KS, Woo HA (2005) Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin, Cell Biol 17:183–189. https://doi.org/10.1016/j.ceb.2005.02.004
de Lores R, Arnaiz G (1990) Effect of tissue specificity of brain soluble fraction on Na K-ATPase Activity. Neurochem Res 15(3):289–294. https://doi.org/10.1007/BF00968674
Santos KL, Vento MA, Wright JW, Speth RC (2013) The effects of para-chloromercuribenzoic acid and different oxidative and sulfhydryl agents on a novel, non-AT1, non-AT2 angiotensin binding site identified as neurolysin. Regul Pept 184:104–114. https://doi.org/10.1016/j.regpep.2013.03.021
Skou J (1957) Influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochem Biophys Acta 23:394–401. https://doi.org/10.1016/0006-3002(57)90343-8
Stadtman ER (1992) Protein oxidation and aging. Science 257:1220–1224. https://doi.org/10.1126/science.1355616
Toyoshima C, Kanai R, Cornelius F (2011) First crystal structures of Na+, K+-ATPase: new light on the oldest ion pump. Structure 19:1732–1738. https://doi.org/10.1016/j.str.2011.10.016
Vague P, Coste TC, Jannot MF, Raccah D, Tsimaratos M (2004) C-peptide, Na K-ATPase and Diabetes. Exp Diabesity Res 5:37–50. https://doi.org/10.1080/15438600490424514
Vizi A, Seregi A (1982) Receptor independent stimulatory effect of noradrenaline on Na, KA-TPase in rat brain homogenate Role of Lipid Peroxadation. Biochem Pharmacol 31(13):2231–2236. https://doi.org/10.1016/0006-2952(82)90106-x
Author information
Authors and Affiliations
Contributions
All co-authors participated in the research and article preparation.
All authors conceived and designed the study. All authors contributed to manuscript revision and approved the final version of the manuscript.
Gvantsa Chkadua—performed the experiments, formulated hypotheses, developed study objectives, defined experimental, statistical, and analytical approaches, analyzed the data, researched the literature, and wrote the paper.
Eka Nozadze—performed the experiments, theoretical calculation, data analysis (statistical or other), and researched the literature.
Leila Tsakadze—conducted the experiments, and contributed to acquisition, analysis and interpretation of the data.
Lia Shioshvili—conducted the experiments, and contributed to acquisition, analysis and interpretation of the data.
Nana Arutinova—performed the experiments, and collected and analyzed the data.
Marine Leladze—performed the experiments, and collected and analyzed the data.
Sopio Dzneladze—performed the experiments, and collected and analyzed the data.
Maia Javakhishvili—performed the experiments, and collected and analyzed the data.
Corresponding author
Ethics declarations
Conflicts of interests
The co-authors of the manuscript have no conflicts of interest to disclose.
Ethics approval
The rats experienced no suffering prior to death, as their death was caused by decapitation. All experiments were approved by the animal care and use committee at the I. Beritashvili Center of Experimental Biomedicine.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chkadua, G., Nozadze, E., Tsakadze, L. et al. Effect of H2O2 on Na,K-ATPase. J Bioenerg Biomembr 54, 241–249 (2022). https://doi.org/10.1007/s10863-022-09948-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-022-09948-1