Abstract
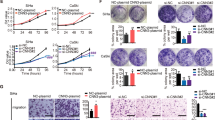
Cervical cancer (CC) is one of the most common malignancy and is the second leading cause of death in gynecologic malignancies worldwide. The homeobox transcription factor homeobox C13 (HOXC13) has been demonstrated to play crucial roles in various cancers. However, its function in CC remains to be addressed. In the present study, upregulation of HOXC13 expression in human CC tissues was found in The Cancer Genome Atlas (TCGA) dataset and clinical samples and was associated with tumor size, FIGO stage and lymph node metastasis. Quantitative real-time polymerase chain reaction (qRT-PCR) and western blot assays suggested that the expression of HOXC13 was up-regulated in CC cells. Cell Counting Kit (CCK)-8, colony formation and cell cycle analysis assays indicated that HOXC13 promoted the proliferation and cycle progression of CC cells in vitro. Of note, knockdown of HOXC13 hinders tumor growth of xenograft tumor mice in vivo. Moreover, transwell and glycolysis measurement assays demonstrated that HOXC13 enhanced the migration, invasion and glycolysis of CC cells in vitro. Further mechanism analysis suggested that HOXC13 participated in CC progression through regulation of the β-catenin/c-Myc signaling pathway. Collectively, HOXC13 facilitated cell proliferation, migration, invasion and glycolysis through modulating β-catenin/c-Myc signaling pathway in CC, indicating that HOXC13 may provide a promising therapeutic target for the therapy of CC.
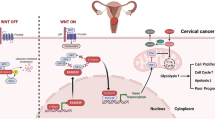
Graphical abstract







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
Code availability
Not Applicable.
References
Bhatlekar S, Fields JZ, Boman BM (2014) HOX genes and their role in the development of human cancers. J Mol Med 92(8):811–823
Bhatlekar S, Fields JZ, Boman BM (2018) Role of HOX genes in stem cell differentiation and cancer. Stem Cells Int 2018:3569493
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Derks M, van der Velden J, de Kroon CD, Nijman HW, van Lonkhuijzen LRCW, van der Zee AGJ, Zwinderman AH, Kenter GG (2018) Surgical treatment of early-stage cervical cancer: a multi-institution experience in 2124 cases in the Netherlands over a 30-year period. Int J Gynecol Cancer 28(4):757
Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, De Robertis EM (2007) Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell 131(5):980–993
Gordon J (2018) Hox genes in the pharyngeal region: How Hoxa3 controls early embryonic development of the pharyngeal organs. Int J Dev Biol 62(11–12):775–783
Gupta A, Ajith A, Singh S, Panday RK, Samaiya A, Shukla S (2018) PAK2–c-Myc–PKM2 axis plays an essential role in head and neck oncogenesis via regulating Warburg effect. Cell Death Dis 9(8):825
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Hasanpourghadi M, YengLooi C, Kumar Pandurangan A, Sethi G, Fen Wong W, Rais Mustafa M (2017) Phytometabolites targeting the Warburg effect in cancer cells: a mechanistic review. J Curr Drug Targets 18(9):1086–1094
Hsieh AL, Walton ZE, Altman BJ, Stine ZE, Dang CV (2015) MYC and metabolism on the path to cancer. Semin Cell Dev Biol 43:11–21
Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, Hild M, Shi X, Wilson CJ, Mickanin C, Myer V, Fazal A, Tomlinson R, Serluca F, Shao W, Cheng H, Shultz M, Rau C, Schirle M, Schlegl J, Ghidelli S, Fawell S, Lu C, Curtis D, Kirschner MW, Lengauer C, Finan PM, Tallarico JA, Bouwmeester T, Porter JA, Bauer A, Cong F (2009) Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461(7264):614–620
Ishii Y, Taguchi A, Kukimoto I (2020) The homeobox transcription factor HOXC13 upregulates human papillomavirus E1 gene expression and contributes to viral genome maintenance. FEBS Lett 594(4):751–762
Kasiri S, Ansari KI, Hussain I, Bhan A, Mandal SS (2013) Antisense oligonucleotide mediated knockdown of HOXC13 affects cell growth and induces apoptosis in tumor cells and over expression of HOXC13 induces 3D-colony formation. J RSC Adv 3(10):3260–3269
Kim J-w, Gao P Liu, Y-C, Semenza GL, Dang CV (2007) HIF-1 and dysregulated c-Myc cooperatively induces VEGF and metabolic switches, HK2 and PDK1. J Mol Cell Biol
Kobayashi Y, Banno K, Kunitomi H, Takahashi T, Takeda T, Nakamura K, Tsuji K, Tominaga E, Aoki D (2019) Warburg effect in gynecologic cancers. J Obstet Gynaecol Res 45(3):542–548
Koppenol WH, Bounds PL, Dang CV (2011) Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer 11(5):325–337
Li C, Cui J, Zou L, Zhu L, Wei W (2020) Bioinformatics analysis of the expression of HOXC13 and its role in the prognosis of breast cancer. J Oncol Lett 19(1):899–907
Li L, Wang Y, Song G, Zhang X, Gao S, Liu H (2019) HOX cluster-embedded antisense long non-coding RNAs in lung cancer. Cancer Lett 450:14–21
Li W, Zhu Q, Zhang S, Liu L, Zhang H, Zhu D (2020) HOXC13-AS accelerates cell proliferation and migration in oral squamous cell carcinoma via miR-378g/HOXC13 axis. Oral Oncol 111:104946
Liberti MV, Locasale JW (2016) The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci 41(3):211–218
Liu F, Chen Y, Zhu G, Hysi PG, Wu S, Adhikari K, Breslin K, Pośpiech E, Hamer MA, Peng F, Muralidharan C, Acuna-Alonzo V, Canizales-Quinteros S, Bedoya G, Gallo C, Poletti G, Rothhammer F, Bortolini MC, Gonzalez-Jose R, Zeng C, Xu S, Jin L, Uitterlinden AG, Ikram MA, van Duijn CM, Nijsten T, Walsh S, Branicki W, Wang S, Ruiz-Linares A, Spector TD, Martin NG, Medland SE, Kayser M (2018) Meta-analysis of genome-wide association studies identifies 8 novel loci involved in shape variation of human head hair. Hum Mol Genet 27(3):559–575
Luo J, Wang Z, Huang J, Yao Y, Sun Q, Wang J, Shen Y, Xu L, Ren B (2018) HOXC13 promotes proliferation of esophageal squamous cell carcinoma via repressing transcription of CASP3. Cancer Sci 109(2):317–329
Luo Z, Rhie SK, Farnham PJJC (2019) The enigmatic HOX genes: can we crack their code? Cancers 11(3):323
Mallo M (2020) The vertebrate tail: a gene playground for evolution. Cell Mol Life Sci 77(6):1021–1030
Meijer CJLM, Snijders PJF (2014) Screening comes of age and treatment progress continues. Nat Rev Clin Oncol 11(2):77–78
Meng H, Liu J, Qiu J, Nie S, Jiang Y, Wan Y, Cheng W (2020) Identification of key genes in association with progression and prognosis in cervical squamous cell carcinoma. DNA Cell Biol 39(5):848–863
Miller DM, Thomas SD, Islam A, Muench D, Sedoris K (2012) c-Myc and cancer metabolism. Clin Cancer Res 18(20):5546
Osthus RC, Shim H, Kim S, Li Q, Reddy R, Mukherjee M, Xu Y, Wonsey D, Lee LA, Dang CV (2000) Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J Biol Chem 275(29):21797–21800
Reynoso-Noverón N, Peña-Nieves A, Rodríguez MO, Mohar-Betancourt A (2017) Cervical cancer epidemiology. In Cervical cancer. Springer, pp 19–33
Shim H, Dolde C, Lewis BC, Wu C-S, Dang G, Jungmann RA, Dalla-Favera R, Dang CV (1997) c-Myc transactivation of LDH-A: Implications for tumor metabolism and growth. Proc Natl Acad Sci U S A 94(13):6658–6663
Shrestha AD, Neupane D, Vedsted P, Kallestrup P (2018) Cervical cancer prevalence, incidence and mortality in low and Middle Income Countries: a systematic review. Asian Pac J Cancer Prev 19(2):319–324
Small W Jr, Bacon MA, Bajaj A, Chuang LT, Fisher BJ, Harkenrider MM, Jhingran A, Kitchener HC, Mileshkin LR, Viswanathan AN (2017) Cervical cancer: a global health crisis. J Cancer 123(13):2404–2412
Svingen T, Tonissen KF (2003) Altered HOX gene expression in human skin and breast cancer cells. Cancer Biol Ther 2(5):518–523
Turcotte M, Abadi A, Peralta-Romero J, Suarez F, Reddon H, Gomez-Zamudio J, Burguete-Garcia AI, Cruz M, Meyre D (2019) Genetic contribution to waist-to-hip ratio in Mexican children and adolescents based on 12 loci validated in European adults. Int J Obes 43(1):13–22
Vander Heiden MG (2011) Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov 10(9):671–684
Warburg O, Wind F, Negelein E (1927) The metabolism of tumors in the body. J Gen Physiol 8(6):519–530
Wu Y, Wang A, Zhu B, Huang J, Lu E, Xu H, Xia W, Dong G, Jiang F, Xu L (2018) KIF18B promotes tumor progression through activating the Wnt/β-catenin pathway in cervical cancer. Onco Targets Ther 11:1707–1720
Yao Y, Luo J, Sun Q, Xu T, Sun S, Chen M, Lin X, Qian Q, Zhang Y, Cao L, Zhang P, Lin Y (2017) HOXC13 promotes proliferation of lung adenocarcinoma via modulation of CCND1 and CCNE1. Am J Cancer Res 7(9):1820–1834
Author information
Authors and Affiliations
Contributions
MMD and LS designed the study, supervised the data collection, analyzed the data,
JJS and LYW interpreted the data and prepared the manuscript for publication, KNZ supervised the data collection, analyzed the data and reviewed the draft of the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All experiments in this study were approved by the Animal Care and Use Committee of the 2nd Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University and performed in accordance with the Guide for the Care and Use of Laboratory Animals.
Consent to participate
Not Applicable.
Consent for publication
Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
Competing interests
The authors state that there are no conflicts of interest to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dai, M., Song, J., Wang, L. et al. HOXC13 promotes cervical cancer proliferation, invasion and Warburg effect through β-catenin/c-Myc signaling pathway. J Bioenerg Biomembr 53, 597–608 (2021). https://doi.org/10.1007/s10863-021-09908-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-021-09908-1