Abstract
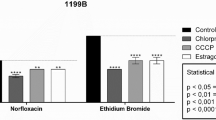
The present study aimed to evaluate the in vitro efflux pump inhibitory capacity of hydroxyamines derived from lapachol and norlachol, where compounds 3, 4, and 5 were tested against the S. aureus strains: RN4220 carrying the pUL5054 plasmid; and IS-58, endowed with the PT181 plasmid. The substances were synthesized from 2-hydroxy-quinones, lapachol and nor-lapachol obtaining the corresponding 2-methoxylated derivatives via dimethyl sulfate alkylation in a basic medium, which then reacted chemoselectively with 2-ethanolamine and 3-propanolamine to form the corresponding amino alcohols. The antibacterial action of the substances was quantified by determining the Minimum Inhibitory Concentration (MIC), while a microdilution assay was carried out to ascertain efflux pump inhibition of Staphylococcus aureus strains carrying the MsrA macrolide and the TetK tetracycline efflux pumps with the substances at a sub-inhibitory concentration. The results were subjected to statistical analysis by an ANOVA test and Bonferroni post hoc test. The MIC from the substances exhibited a value ≥ 1024 µg/mL. However, a significant reduction (p < 0.0001) of the erythromycin, tetracycline and ethidium bromide MIC was demonstrated when these were in combination with the substances, with this effect being due to a supposed efflux pump inhibition. The tested substances demonstrated effectiveness at decreasing the MIC of erythromycin, tetracycline and ethidium bromide, potentially by inhibiting the MsrA macrolide and the TetK tetracycline efflux pumps present in the tested S. aureus strains.

Similar content being viewed by others
References
Barbosa TP, Camara CA, Silva TMS, Martins RM, Pinto AC, Vargas MD (2005) New 1,2,3,4-tetrahydro-1-aza-anthraquinones and 2-aminoalkyl compounds from nor-lapachol with molluscicidal activity. Bioorg Med Chem 13(2005):6464–6469. https://doi.org/10.1016/j.bmc.2005.06.068
Barreto HM, Fontinele FC, Oliveira AP, Arcanjo DDR, Santos BHC, Abreu APL et al (2014) Phytochemical prospection and modulation of antibiotic activity in vitro by Lippia origanoides H.B.K. In: Methicillin Resistant Staphylococcus aureus. BioMed Res Int 2014:1–7
Camara CA, Pinto AC, Vargas MD, Zukerman-Schpector J (2002) Azepines from the intramolecular prins cyclization of an aminoderivative of lapachol. Tetrahedron 58(30):6135–6140. https://doi.org/10.1016/S0040-4020(02)00581-1
Cavalcanti BC, Cabral IO, Rodrigues FAR, Barros FWA, Rocha DD, Magalhães HIF, Moura DJ, Saffi J, Henriques JAP, Carvalho TSC, Moraes MO, Pessoa C, Melo IMM, Júnior ENS (2013) Potent antileukemic action of naphthoquinoidal compounds: evidence for an intrinsic death mechanism based on oxidative stress and inhibition of DNA repair. J Braz Chem Soc 24(1):145–163. https://doi.org/10.1590/S0103-50532013000100019
CLSI (2013) Performance standards for antimicrobial susceptibility testing; Twentythird informational supplement. CLSI document M100eS23.Publishing PhysicsWeb. https://clsi.org/search/?q=Performance+standards+for+antimicrobial+susceptibility+testing%3B+Twentythird+informational+supplement.+CLSI+document+M100eS23. Accessed 12 May 2019
Costa SS, Viveiros M, Amaral L, Couto I (2013) Multidrug efflux pumps in staphylococcus aureus: an update. Open Microbiol J 7:59–71
Coutinho HDM, Costa JG, Lima EO, Falcão-Silva VS, Siqueira-Júnior JP (2008) Enhancement of the antibiotic activity against a multiresistant Escherichia coli by Mentha arvensis L. and chlorpromazine. Chemotherapy 54:328–330
Coutinho HDM, Costa JGM, Lima EO, Siqueira-Júnior JP (2010) Additive effects of Hyptis martiusii Benth with aminoglycosides against Escherichia coli. Indian J Med Res 131:106–108
Couto I, Costa SS, Viveiros M, Martins M, Amaral L (2008) Efflux-mediated response of Staphylococcus aureus exposed to ethidium bromide. J Antimicrob Chemother 62(3):504–513
Daferera DJ, Ziogas BN, Polissiou MG (2003) The effectiveness of plant essential oils on the growth of Botrytis cinerea, Fusariumsp. and Clavibactermichiganensissub sp. michiganensis. Crop Prot 22:39–44
DeMarco CE, Cushing LA, Frempong-Manso E, Seo SM, Jaravaza TAA, Kaatz GW (2007) Efflux-related resistance to norfloxacin, dyes and biocides in bloodstream isolates of Staphylococcus aureus. Antimicrob Agents Chemother 51:3235-3239. https://doi.org/10.1128/AAC.00430-07
Farias PAM, Figueredo FG, Lucas AMB, Moura RB, Coutinho HDM, Silva TMS, Martin ALAR, Fonteles MMF (2015) Antiobiotic-modifying activity of riachin, a non-cyanogenic cyanoglycoside extracted from Bauinia pentandra. Drug Des Devel Ther 9:3067–3072
Ferreira SB, Gonzaga DTG, Santos WC, Araújo KGL, Ferreira VF (2010) β-Lapachona: Sua importância em química medicinal e modificações estruturais. Rev Virtual Quím 2(2):140–160
Figueredo FG, Ramos ITL, Paz JA, Silva, Tania MS, Camara CA, Oliveira-Tintino CDM, Tintino SR, Farias PAM, Coutinho HDM, Fonteles MMF (2020a) In silico evaluation of the antibacterial and modulatory activity of lapachol and nor-lapachol DERIVATES. Microb Pathog 144:104181
Figueredo FG, Ramos IT, Paz JA, Farias PAM, ; SILVA TMS, Camara CA, Tintino SR, Menezes IA, Coutinho HDM, Fonteles MMF (2020) Effect of hydroxyamines derived from lapachol and norlachol against Staphylococcus aureus strains carrying the NorA efflux pump. Infect Genet Evol 84:104370. https://doi.org/10.1016/j.meegid.2020.104370
Hal SJV, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB (2012) Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev 25(2):362–386
Abd El-Kalek HH, Mohamed EA (2012) Synergistic effect of certain medicinal plants and amoxicillin against some clinical isolates of methicillin – resistant Staphylococcus Aureus (MRSA). Int J Pharmaceut Appl 3:387–398
Houghton PJ, Howes MJ, Lee CC, Steventon G (2007) Uses and abuses of in vitro tests in ethnopharmacology: visualizing an elephant. J Ethnopharmacol 110(3):391–400
Júnior ENS (2007) Síntese de novos derivados de lapachonas e nor-laopachonas: Veredas à atividade farmacológica. Dissertation, Universidade Federal do Rio de Janeiro
Kaatz GW, Moudgal VV, Seo SM, Kristiansen JE (2003) Phenothiazines and thioxanthenes inhibit multidrug efflux pump activity in Staphylococcus aureus. Antimicrob Agents Chemother 47(2):719–726
Kristiansen J, Mortensen I, Nissen B (1992) Membrane stabilizers inhibit potassium efflux from Staphylococcus aureus strain no. U2275. Biochem Biophys Acta 685:82–379
Kullar R, Sakoulas G, Deresinski S, Hal SJV (2016) When sepsis persists: a review of MRSA bacteraemia salvage therapy. J Antimicrob Chemother 71(3):86–576
Lamers RP, Cavallari JF, Burrows LL (2013) The efflux inhibitor Phenylalanine-Arginine Beta-Naphthylamide (PAβN) permeabilizes the outer membrane of gram-negative bacteria. PLoS One 8(3):1–7
Laudy AE, Osińska P, Namysłowska A, Zając O, Tyski S (2015) Modification of the susceptibility of gram-negative rods producing ESβLS to β-lactams by the efflux phenomenon. PLoS One 10(3):1–14
Lima WG, Ramos-Alves MC, Soares AC (2019) Dos distúrbios psiquiátricos à antibioticoterapia: reposicionamento da clorpromazina como agente antibacteriano. Rev Colomb Cienc Quím Farm 48(1):5–28
Limaverde PW, Campina FF, Cunha FABD, Crispim FD, Figueredo FG, Lima LF, Oliveira-Tintino CD, Matos YMLS, Morais-Braga MFB, Menezes IRA, Balbino VQ, Coutinho HDM, Siqueira-Júnior JP, Almeida JRGS, Tintino SR (2017) Inhibition of the TetK efflux-pump by the essential oil of Chenopodium ambrosioides L. and α-terpinene against Staphylococcus aureus IS-58. Food Chem Toxicol 17:1–18
Lomovskaya O, Warren MS, Lee A, Galazzo J, Fronko R, Lee M et al (2001) Identification and characterization of inhibitors of multidrug resistance efflux pumps in pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob Agents Chemother 45(1):105–116
Lowy FD (2019) Methicillin-resistant Staphylococcus aureus (MRSA): Microbiology. IOP Publishing PhysicsWeb. https://www.uptodate.com/contents/methicillin-resistant-staphylococcus-aureus-mrsa-microbiology/print?search=staphylococcus%20aureus&topicRef=3157&source=see_link. Accessed 16 Sep 2019
Monaco M, Araujo FPD, Cruciani M, Coccia EM, Pantosti A (2017) Worldwide epidemiology and antibiotic resistance of Staphylococcus aureus. Curr Top Microbiol Immunol 409:21–56
Murray KP, Zhao J, Davis SL, Kullar R, Kaye K, Lephart P et al (2013) Early use of daptomycin versus vancomycin for methicillin-resistant Staphylococcus aureus bacteremia with vancomycin minimum inhibitory concentration > 1 mg/L: a matched cohort study. Clin Infect Dis 56(11):9–1562
Oliveira FQ, Gobira B, Guimarães C, Batista J, Barreto M, Souza, M2007a. Espécies vegetais indicadas na odontologia. Rev Bras Farmacognosia 17:466–476
Olmsted J III, Kearns DR (1977) Mechanism of ethidium bromide fluorescence enhancement on binding to nucleic acids. Biochemistry 16(16):3647–3654
Pantosti A, Sanchini A, Monaco M (2007) Mechanisms of antibiotic resistance in Staphylococcus aureus. Future Microbiol 2(3):323–334
Patel D, Kosmidis C, Seo SM, Kaatz GW (2010) Ethidium bromide MIC screening for enhanced efflux pump gene expression or efflux activity in Staphylococcus aureus. Antimicrob Agents Chemother 54(12):73–5070
Piddock LJ (2006) Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin Microbiol Rev 19:382–402
Powis G (1989) Free radical formation by antitumor quinones. Free Radic Biol Med 6:63–105. https://doi.org/10.1016/0891-5849(89)90162-7
Schuster S, Bohnert JA, Vavra M, Rossen JW, Kern WV (2019) Proof of an outer membrane target of the efflux inhibitor Phe-Arg-β-Naphthylamide from random mutagenesis. Molecules 24(3):1–12
Shurland S, Zhan M, Bradham DD, Roghmann MC (2007) Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol 28(3):9–273
Silva MN, Ferreira VF, Souza MCBV (2003) Um panorama atual da química e da farmacologia de naftoquinonas, com ênfase na β-lapachona e derivados. Quím Nova 26:407–416
Silva JG, Souza IA, Higino JS, Siqueira-Junior JP, Pereira JV, Pereira MSV (2007) Atividade antimicrobiana do extrato de AnacardiumoccidentaleLinn. Em amostras multiresistentes de Staphylococcus aureus. Rev Bras Farmacognosia 7:572–577
Suthanan SN, Veale CGL, Gounden NS, Osoniyi O, Hendricks DT, Caira MR, La Mare JA, Edkins AL, Pinto AV, Júnior E N. DA S., Coleman MTD (2013) Cytotoxicity of lapachol, β-lapachone and related synthetic 1,4- naphthoquinones against oesophageal cancer cells. Eur J Med Chem 62:98–100
Taylor TA, Unakal CG. Staphylococcus aureus. StatPearls [Internet]: IOP Publishing PhysicsWeb. https://www.ncbi.nlm.nih.gov/books/NBK441868/. Accessed 16 Sep 2019
Truong-Bolduc Q, Dunman PM, Strahilevitz J, Projan SJ, Hooper DC (2005) MgrA é um regulador múltiplo de duas novas bombas de efluxo em Staphylococcus aureus. J Bacteriol 187(7):2395–2405
Van BF, Pages JM, Lee VJ (2006) Inhibitors of bacterial efflux pumps as adjuvants in antibiotic treatments and diagnostic tools for detection of resistance by efflux. Recent Pat Antiinfect Drug Discov 1:157–175
Vance GFJ, Holland TL (2008) Clinical approach to Staphylococcus aureus bacteremia in adults. IOP Publishing PhysicsWeb. https://www.uptodate.com/contents/clinical-approach-to-staphylococcus-aureus-bacteremia-in-adults. Accessed 14 Sep 2019
Viveiros M, Martins A, Paixão L, Rodrigues L, Martins M, Couto I et al (2008) Demonstration of intrinsic efflux activity of escherichia coli K-12 AG100 by an automated ethidium bromide method. Int J Antimicrob Agents 31(5):62–458
Wagner H, Ulrich-Merzenich G (2009) Synergy research: approaching a new generation of phytopharmaceuticals. Phytomedicine 16:97–110
Wertheim HFL, Melles DC, Vos MC, Leeuwen WV, Belkum AV, Verbrugh HA, Nouwen JL (2005) The role of nasal carriage in Staphylococcus aureusinfections. Lancet Infect Dis 5(12):62–751
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Figueredo, F.G., Parente, R.E.L.T., Cavalcante-Figueredo, M.R. et al. Inhibition of Staphylococcus aureus TetK and MsrA efflux pumps by hydroxyamines derived from lapachol and norlachol. J Bioenerg Biomembr 53, 149–156 (2021). https://doi.org/10.1007/s10863-021-09885-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-021-09885-5