Abstract
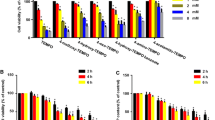
A compound with promising anticancer properties, 3-bromopyruvate (3-BP) is a synthetic derivative of a pyruvate molecule; however, its toxicity in non-malignant cells has not yet been fully elucidated. Therefore, we elected to study the effects of 3-BP on primary hepatocytes in monolayer cultures, permeabilized hepatocytes and isolated mitochondria. After a 1-h treatment with 100 μM 3-BP cell viability of rat hepatocytes was decreased by 30 % as measured by the WST-1 test (p < 0.001); after 3-h exposure to ≥200 μM 3-BP lactate dehydrogenase leakage was increased (p < 0.001). Reactive oxygen species production was increased in the cell cultures after a 1-h treatment at concentrations ≥100 μmol/l (p < 0.01), and caspase 3 activity was increased after a 20-h incubation with 150 μM and 200 μM 3-BP (p < 0.001). This toxic effect of 3-BP was also proved using primary mouse hepatocytes. In isolated mitochondria, 3-BP induced a dose- and time-dependent decrease of mitochondrial membrane potential during a 10-min incubation both with Complex I substrates glutamate + malate or Complex II substrate succinate, although this decrease was more pronounced with the latter. We also measured the effect of 3-BP on respiration of isolated mitochondria. ADP-activated respiration was inhibited by 20 μM 3-BP within 10 min. Similar effects were also found in permeabilized hepatocytes of both species.







Similar content being viewed by others
References
Akerman KE, Wikstrom MK (1976) Safranine as a probe of the mitochondrial membrane potential. FEBS Lett 68:191–197
Berry MN, Edwards AM, Barritt GJ (1991) High-yield preparation of isolated hepatocytes from rat liver. In: MN B (ed) Isolated Hepatocytes Preparation, Properties and Application,, 1st edn. Elsevier, New York, pp. 15–81
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cardaci S, Desideri E, Ciriolo MR (2012) Targeting aerobic glycolysis: 3-bromopyruvate as a promising anticancer drug. J Bioenerg Biomembr 44:17–29. doi:10.1007/s10863-012-9422-7
Chen Z, Zhang H, Lu W, Huang P (2009) Role of mitochondria-associated hexokinase II in cancer cell death induced by 3-bromopyruvate. Biochim Biophys Acta 1787:553–560. doi:10.1016/j.bbabio.2009.03.003
Dell’Antone P (2009) Targets of 3-bromopyruvate, a new, energy depleting, anticancer agent. Med Chem 5:491–496
Drahota Z, Endlicher R, Stankova P, Rychtrmoc D, Milerova M, Cervinkova Z (2012) Characterization of calcium, phosphate and peroxide interactions in activation of mitochondrial swelling using derivative of the swelling curves. J Bioenerg Biomembr 44:309–315. doi:10.1007/s10863-012-9443-2
Ehrke E, Arend C, Dringen R (2015) 3-bromopyruvate inhibits glycolysis, depletes cellular glutathione, and compromises the viability of cultured primary rat astrocytes. J Neurosci Res 93:1138–1146. doi:10.1002/jnr.23474
El Sayed SM et al. (2012) D-amino acid oxidase gene therapy sensitizes glioma cells to the antiglycolytic effect of 3-bromopyruvate. Cancer Gene Ther 19:1–18. doi:10.1038/cgt.2011.59
Geschwind JF, Ko YH, Torbenson MS, Magee C, Pedersen PL (2002) Novel therapy for liver cancer: direct intraarterial injection of a potent inhibitor of ATP production. Cancer Res 62:3909–3913
Griss T et al. (2015) Metformin Antagonizes Cancer Cell Proliferation by Suppressing Mitochondrial-Dependent Biosynthesis. PLoS Biol 13:e1002309. doi:10.1371/journal.pbio.1002309
Jae HJ et al. (2009) The antitumor effect and hepatotoxicity of a hexokinase II inhibitor 3-bromopyruvate: in vivo investigation of intraarterial administration in a rabbit VX2 hepatoma model. Korean J Radiol 10:596–603. doi:10.3348/kjr.2009.10.6.596
Kim JS et al. (2008) Role of reactive oxygen species-mediated mitochondrial dysregulation in 3-bromopyruvate induced cell death in hepatoma cells : ROS-mediated cell death by 3-BrPA. J Bioenerg Biomembr 40:607–618. doi:10.1007/s10863-008-9188-0
Kluckova K et al. (2015) Ubiquinone-binding site mutagenesis reveals the role of mitochondrial complex II in cell death initiation. Cell Death Dis 6:e1749. doi:10.1038/cddis.2015.110
Ko YH, Pedersen PL, Geschwind JF (2001) Glucose catabolism in the rabbit VX2 tumor model for liver cancer: characterization and targeting hexokinase. Cancer Lett 173:83–91
Ko YH et al. (2004) Advanced cancers: eradication in all cases using 3-bromopyruvate therapy to deplete ATP. Biochem Biophys Res Commun 324:269–275. doi:10.1016/j.bbrc.2004.09.047
Ko YH, Verhoeven HA, Lee MJ, Corbin DJ, Vogl TJ, Pedersen PL (2012) A translational study "case report" on the small molecule "energy blocker" 3-bromopyruvate (3BP) as a potent anticancer agent: from bench side to bedside. J Bioenerg Biomembr 44:163–170. doi:10.1007/s10863-012-9417-4
Kucera O, Al-Dury S, Lotkova H, Rousar T, Rychtrmoc D, Cervinkova Z (2012) Steatotic rat hepatocytes in primary culture are more susceptible to the acute toxic effect of acetaminophen. Physiol Res 61(Suppl 2):S93–101
Kucera O, Endlicher R, Rousar T, Lotkova H, Garnol T, Drahota Z, Cervinkova Z (2014) The effect of tert-butyl hydroperoxide-induced oxidative stress on lean and steatotic rat hepatocytes in vitro. Oxidative Med Cell Longev 2014:752506. doi:10.1155/2014/752506
Kunjithapatham R, Geschwind JF, Rao PP, Boronina TN, Cole RN, Ganapathy-Kanniappan S (2013) Systemic administration of 3-bromopyruvate reveals its interaction with serum proteins in a rat model. BMC Res Notes 6:277. doi:10.1186/1756-0500-6-277
Kwiatkowska E, Wojtala M, Gajewska A, Soszynski M, Bartosz G, Sadowska-Bartosz I (2016) Effect of 3-bromopyruvate acid on the redox equilibrium in non-invasive MCF-7 and invasive MDA-MB-231 breast cancer cells. J Bioenerg Biomembr 48:23–32. doi:10.1007/s10863-015-9637-5
Mezera V et al. (2015) Comparison of acetaminophen toxicity in primary hepatocytes isolated from transgenic mice with different appolipoprotein E alleles journal of physiology and pharmacology: an official journal of the polish physiological. Society 66:863–873
Neuzil J et al. (2007) Vitamin E analogues as a novel group of mitocans: anti-cancer agents that act by targeting mitochondria. Mol Asp Med 28:607–645. doi:10.1016/j.mam.2007.02.003
Pecinova A, Drahota Z, Nuskova H, Pecina P, Houstek J (2011) Evaluation of basic mitochondrial functions using rat tissue homogenates. Mitochondrion 11:722–728. doi:10.1016/j.mito.2011.05.006
Pedersen PL (2007a) The cancer cell’s "power plants" as promising therapeutic targets: an overview. J Bioenerg Biomembr 39:1–12. doi:10.1007/s10863-007-9070-5
Pedersen PL (2007b) Warburg, me and Hexokinase 2: Multiple discoveries of key molecular events underlying one of cancers’ most common phenotypes, the "Warburg Effect", i.e., elevated glycolysis in the presence of oxygen. J Bioenerg Biomembr 39:211–222. doi:10.1007/s10863-007-9094-x
Pereira da Silva AP et al. (2009) Inhibition of energy-producing pathways of HepG2 cells by 3-bromopyruvate. Biochem J 417:717–726. doi:10.1042/bj20080805
Pesta D, Gnaiger E (2012) High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol 810:25–58. doi:10.1007/978-1-61779-382-0_3
Rodrigues-Ferreira C, da Silva AP, Galina A (2012) Effect of the antitumoral alkylating agent 3-bromopyruvate on mitochondrial respiration: role of mitochondrially bound hexokinase. J Bioenerg Biomembr 44:39–49. doi:10.1007/s10863-012-9413-8
Sadowska-Bartosz I, Bartosz G (2013) Effect of 3-bromopyruvic acid on human erythrocyte antioxidant defense system. Cell Biol Int 37:1285–1290. doi:10.1002/cbin.10160
Sanborn BM, Felberg NT, Hollocher TC (1971) The inactivation of succinate dehydrogenase by bromopyruvate. Biochim Biophys Acta 227:219–231
Shoshan MC (2012) 3-Bromopyruvate: targets and outcomes. J Bioenerg Biomembr 44:7–15. doi:10.1007/s10863-012-9419-2
Sun Y et al. (2015) Mechanisms underlying 3-bromopyruvate-induced cell death in colon cancer. J Bioenerg Biomembr 47:319–329. doi:10.1007/s10863-015-9612-1
Warburg O (1956) On respiratory impairment in cancer cells. Science 124:269–270
Warmoes MO, Locasale JW (2014) Heterogeneity of glycolysis in cancers and therapeutic opportunities. Biochem Pharmacol 92:12–21. doi:10.1016/j.bcp.2014.07.019
Wu L et al. (2014) The reversal effects of 3-bromopyruvate on multidrug resistance in vitro and in vivo derived from human breast MCF-7/ADR cells. PLoS One 9:e112132. doi:10.1371/journal.pone.0112132
Xian SL, Cao W, Zhang XD, YF L (2015) 3-bromopyruvate inhibits human gastric cancer tumor growth in nude mice via the inhibition of glycolysis. Oncol Lett 9:739–744. doi:10.3892/ol.2014.2779
Xintaropoulou C, Ward C, Wise A, Marston H, Turnbull A, Langdon SP (2015) A comparative analysis of inhibitors of the glycolysis pathway in breast and ovarian cancer cell line models. Oncotarget 6:25677–25695. doi:10.18632/oncotarget.4499
Xu RH et al. (2005) Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res 65:613–621
Xu DQ, Tan XY, Zhang BW, Wu T, Liu P, Sun SJ, Cao YG (2015) 3-Bromopyruvate inhibits cell proliferation and induces apoptosis in CD133+ population in human glioma. Tumour Biol. doi:10.1007/s13277-015-3884-2
Zhang Q, Pan J, North PE, Yang S, Lubet RA, Wang Y, You M (2012) Aerosolized 3-bromopyruvate inhibits lung tumorigenesis without causing liver toxicity. Cancer Prev Res (Phila) 5:717–725. doi:10.1158/1940-6207.capr-11-0338
Zhang Q, Pan J, Lubet RA, Komas SM, Kalyanaraman B, Wang Y, You M (2015) Enhanced antitumor activity of 3-bromopyruvate in combination with rapamycin in vivo and in vitro. Cancer Prev Res (Phila) 8:318–326. doi:10.1158/1940-6207.capr-14-0142
Acknowledgments
This work was supported by grants PRVOUK P37/02 and SVV-2015-260179 from Charles University in Prague.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sobotka, O., Endlicher, R., Drahota, Z. et al. Impaired mitochondrial functions contribute to 3-bromopyruvate toxicity in primary rat and mouse hepatocytes. J Bioenerg Biomembr 48, 363–373 (2016). https://doi.org/10.1007/s10863-016-9674-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-016-9674-8