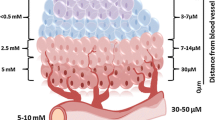
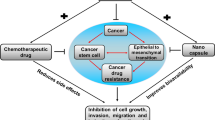
Abstract
At the beginning of the twenty-first century, 3-bromopyruvate (3BP), a simple alkylating chemical compound was presented to the scientific community as a potent anticancer agent, able to cause rapid toxicity to cancer cells without bystander effects on normal tissues. The altered metabolism of cancers, an essential hallmark for their progression, also became their Achilles heel by facilitating 3BP’s selective entry and specific targeting. Treatment with 3BP has been administered in several cancer type models both in vitro and in vivo, either alone or in combination with other anticancer therapeutic approaches. These studies clearly demonstrate 3BP’s broad action against multiple cancer types. Clinical trials using 3BP are needed to further support its anticancer efficacy against multiple cancer types thus making it available to more than 30 million patients living with cancer worldwide. This review discusses current knowledge about 3BP related to cancer and discusses also the possibility of its use in future clinical applications as it relates to safety and treatment issues.



Similar content being viewed by others
References
Amoêdo ND, Valencia JP, Rodrigues MF, Galina A, Rumjanek FD (2013) How does the metabolism of tumour cells differ from that of normal cells. Biosci Rep 33(6). doi:10.1042/BSR20130066
Azevedo-Silva J et al. (2015) The cytotoxicity of 3-Bromopyruvate in breast cancer cells depends on extracellular pH. Biochem J. doi:10.1042/BJ20140921
Baker JP, Rabin BR (1969) Effects of bromopyruvate on the control and catalytic properties of glutamate dehydrogenase. Eur J Biochem 11:154–159
Baltazar F, Pinheiro C, Morais-Santos F, Azevedo-Silva J, Queiros O, Preto A, Casal M (2014) Monocarboxylate transporters as targets and mediators in cancer therapy response. Histol Histopathol 29:1511–1524
Barnard JP, Reynafarje B, Pedersen PL (1993) Glucose catabolism in African trypanosomes. Evidence that the terminal step is catalyzed by a pyruvate transporter capable of facilitating uptake of toxic analogs. J Biol Chem 268:3654–3661
Bean JF, Qiu YY, Yu S, Clark S, Chu F, Madonna MB (2014) Glycolysis inhibition and its effect in doxorubicin resistance in neuroblastoma. J Pediatr Surg 49:981–984; discussion 984 doi:10.1016/j.jpedsurg.2014.01.037
Bhardwaj V, Rizvi N, Lai MB, Lai JC, Bhushan A (2010) Glycolytic enzyme inhibitors affect pancreatic cancer survival by modulating its signaling and energetics. Anticancer Res 30:743–749
Birsoy K et al. (2013) MCT1-mediated transport of a toxic molecule is an effective strategy for targeting glycolytic tumors. Nat Genet 45:104–108. doi:10.1038/ng.2471
Bricker DK et al. (2012) A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science 337(6090): 96–100. doi:10.1126/science.1218099
Buijs M et al. (2009) Specificity of the anti-glycolytic activity of 3-bromopyruvate confirmed by FDG uptake in a rat model of breast cancer. Invest New Drugs 27:120–123. doi:10.1007/s10637-008-9145-0
Buijs M, Wijlemans JW, Kwak BK, Ota S, Geschwind JF (2013) Antiglycolytic therapy combined with an image-guided minimally invasive delivery strategy for the treatment of breast cancer J Vasc Interv Radiol 24:737–743 doi:10.1016/j.jvir.2013.01.013
Byrne FL et al. (2014) Metabolic vulnerabilities in endometrial cancer. Cancer Res 74:5832–5845. doi:10.1158/0008-5472.CAN-14-0254
Calviño E et al. (2014) Regulation of death induction and chemosensitizing action of 3-bromopyruvate in myeloid leukemia cells: energy depletion, oxidative stress, and protein kinase activity modulation. J Pharmacol Exp Ther 348:324–335. doi:10.1124/jpet.113.206714
Cao X et al. (2008) Non-invasive MRI tumor imaging and synergistic anticancer effect of HSP90 inhibitor and glycolysis inhibitor in RIP1-Tag2 transgenic pancreatic tumor model. Cancer Chemother Pharmacol 62:985–994. doi:10.1007/s00280-008-0688-8
Cardaci S et al. (2012) Glutamine deprivation enhances antitumor activity of 3-bromopyruvate through the stabilization of monocarboxylate transporter-1. Cancer Res 72:4526–4536. doi:10.1158/0008-5472.CAN-12-1741
Chang GG, Hsu RY (1973) The substrate analog bromopyruvate as a substrate, an inhibitor and an alkylating agent of malic enzyme of pigeon liver. Biochem Biophys Res Commun 55:580–587
Chang JM, Chung JW, Jae HJ, Eh H, Son KR, Lee KC, Park JH (2007) Local toxicity of hepatic arterial infusion of hexokinase II inhibitor, 3-bromopyruvate: In vivo investigation in normal rabbit model. Acad Radiol 14:85–92. doi:10.1016/j.acra.2006.09.059
Chapiro J et al. (2014) Systemic delivery of microencapsulated 3-bromopyruvate for the therapy of pancreatic cancer. Clin Cancer Res 20:6406–6417. doi:10.1158/1078-0432.CCR-14-1271
Chen Z, Zhang H, Lu W, Huang P (2009) Role of mitochondria-associated hexokinase II in cancer cell death induced by 3-bromopyruvate. Biochim Biophys Acta 1787:553–560. doi:10.1016/j.bbabio.2009.03.003
Davidescu M et al. (2012) Bromopyruvate mediates autophagy and cardiolipin degradation to monolyso-cardiolipin in GL15 glioblastoma cells. J Bioenerg Biomembr 44:51–60. doi:10.1007/s10863-012-9411-x
Davidescu M, Macchioni L, Scaramozzino G, Cristina Marchetti M, Migliorati G, Vitale R, Corcelli A, Roberti R, Castigli E, Corazzi L (2015) The energy blockers bromopyruvate and lonidamine lead GL15 glioblastoma cells to death by different p53-dependent routes. Sci Rep 5:14343. doi:10.1038/srep14343
Dell’Antone P (2006) Inactivation of H + −vacuolar ATPase by the energy blocker 3-bromopyruvate, a new antitumour agent. Life Sci 79:2049–2055. doi:10.1016/j.lfs.2006.06.043
Dell’Antone P (2009) Targets of 3-bromopyruvate, a new, energy depleting, anticancer agent. Med Chem 5:491–496
Donohoe DR, Collins LB, Wali A, Bigler R, Sun W, Bultman SJ (2012) The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol Cell 48:612–626. doi:10.1016/j.molcel.2012.08.033
Dylag M, Lis P, Niedzwiecka K, Ko YH, Pedersen PL, Goffeau A, Ulaszewski S (2013) 3-Bromopyruvate: a novel antifungal agent against the human pathogen Cryptococcus neoformans. Biochem Biophys Res Commun 434:322–327. doi:10.1016/j.bbrc.2013.02.125
Ehrke E, Arend C, Dringen R (2014) 3-bromopyruvate inhibits glycolysis, depletes cellular glutathione, and compromises the viability of cultured primary rat astrocytes. J Neurosci Res. doi:10.1002/jnr.23474
El Sayed SM et al. (2012a) D-amino acid oxidase gene therapy sensitizes glioma cells to the antiglycolytic effect of 3-bromopyruvate. Cancer Gene Ther 19:1–18. doi:10.1038/cgt.2011.59
El Sayed SM et al. (2012b) 3-Bromopyruvate antagonizes effects of lactate and pyruvate, synergizes with citrate and exerts novel anti-glioma effects. J Bioenerg Biomembr 44:61–79. doi:10.1007/s10863-012-9409-4
El Sayed SM et al. (2012c) D-Amino acid oxidase-induced oxidative stress, 3-bromopyruvate and citrate inhibit angiogenesis, exhibiting potent anticancer effects. J Bioenerg Biomembr 44:513–523. doi:10.1007/s10863-012-9455-y
El Sayed SM et al. (2014) Safety and outcome of treatment of metastatic melanoma using 3-bromopyruvate: a concise literature review and case study. Chin J Cancer 33:356–364. doi:10.5732/cjc.013.10111
Galina A (2014) Mitochondria: 3-bromopyruvate vs. mitochondria? A small molecule that attacks tumors by targeting their bioenergetic diversity. Int J Biochem Cell Biol 54:266–271. doi:10.1016/j.biocel.2014.05.013
Ganapathy V, Thangaraju M, Gopal E, Martin PM, Itagaki S, Miyauchi S, Prasad PD (2008) Sodium-coupled monocarboxylate transporters in normal tissues and in cancer. AAPS J 10:193–199. doi:10.1208/s12248-008-9022-y
Ganapathy-Kanniappan S et al. (2010a) 3-Bromopyruvate induces endoplasmic reticulum stress, overcomes autophagy and causes apoptosis in human HCC cell lines. Anticancer Res 30:923–935
Ganapathy-Kanniappan S et al. (2010b) ) 3-bromopyruvate: a new targeted antiglycolytic agent and a promise for cancer therapy. Curr Pharm Biotechnol 11:510–517
Ganapathy-Kanniappan S et al. (2012) Human hepatocellular carcinoma in a mouse model: assessment of tumor response to percutaneous ablation by using glyceraldehyde-3-phosphate dehydrogenase antagonists. Radiology 262:834–845. doi:10.1148/radiol.11111569
Ganapathy-Kanniappan S, Kunjithapatham R, Geschwind JF (2013) Anticancer efficacy of the metabolic blocker 3-bromopyruvate: specific molecular targeting. Anticancer Res 33:13–20
Garbutcheon-Singh KB, Harper BW, Myers S, Aldrich-Wright JR (2014) Combination studies of platinum(II)-based metallointercalators with buthionine-S,R-sulfoximine, 3-bromopyruvate, cisplatin or carboplatin Metallomics : integrated biometal science 6:126–131 doi:10.1039/c3mt00191a
Geschwind JF, Ko YH, Torbenson MS, Magee C, Pedersen PL (2002) Novel therapy for liver cancer: direct intraarterial injection of a potent inhibitor of ATP production. Cancer Res 62:3909–3913
Glick M, Biddle P, Jantzi J, Weaver S, Schirch D (2014) The antitumor agent 3-bromopyruvate has a short half-life at physiological conditions. Biochem Biophys Res Commun 452:170–173. doi:10.1016/j.bbrc.2014.08.066
Gong L, Wei Y, Yu X, Peng J, Leng X (2014) 3-Bromopyruvic acid, a hexokinase II inhibitor, is an effective antitumor agent on the hepatoma cells: in vitro and in vivo findings. Anti Cancer Agents Med Chem 14:771–776
Gothe PO, Nyman PO (1972) Inactivation of human erythrocyte carbonic anhydrases by bromopyruvate. FEBS Lett 21:159–164
Guaragnella N, Giannattasio S, Moro L (2014) Mitochondrial dysfunction in cancer chemoresistance. Biochem Pharmacol 92(1):62–72. doi:10.1016/j.bcp.2014.07.027
Guo C, Liu S, Sun MZ (2013) Novel insight into the role of GAPDH playing in tumor Clinical & Transl Oncol: Official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico 15:167–172 doi:10.1007/s12094-012-0924-x
Guo X, Zhang X, Xian S, Tan F, Lu Y, Wang T (2016) 3-Bromopyruvate and sodium citrate induce apoptosis in human gastric cancer cell line MGC-803 by inhibiting glycolysis and promoting mitochondria-regulated apoptosis pathway. Biochem Biophys Res Commun 475(1):37–43. doi:10.1016/j.bbrc.2016.04.151
Hajdu SI (2011) A note from history: landmarks in history of cancer, part 1. Cancer 117:1097–1102. doi:10.1002/cncr.25553
Herzig S et al. (2012) Identification and functional expression of the mitochondrial pyruvate carrier. Science 337:93–96. doi:10.1126/science.1218530
Higashimura Y, Nakajima Y, Yamaji R, Harada N, Shibasaki F, Nakano Y, Inui H (2011) Up-regulation of glyceraldehyde-3-phosphate dehydrogenase gene expression by HIF-1 activity depending on Sp1 in hypoxic breast cancer cells. Arch Biochem Biophys 509:1–8. doi:10.1016/j.abb.2011.02.011
Hulleman E et al. (2009) Inhibition of glycolysis modulates prednisolone resistance in acute lymphoblastic leukemia cells. Blood 113:2014–2021. doi:10.1182/blood-2008-05-157842
Hussien R, Brooks GA (2011) Mitochondrial and plasma membrane lactate transporter and lactate dehydrogenase isoform expression in breast cancer cell lines. Physiol Genomics 43(5):255–64. doi:10.1152/physiolgenomics.00177.2010
Icard P, Zhang XD, Lemoisson E, Louis MH, Allouche S, Lincet H, Poulain L (2012) Experimental results using 3-bromopyruvate in mesothelioma: in vitro and in vivo studies. J Bioenerg Biomembr 44:81–90. doi:10.1007/s10863-012-9415-6
Ihrlund LS, Hernlund E, Khan O, Shoshan MC (2008) 3-Bromopyruvate as inhibitor of tumour cell energy metabolism and chemopotentiator of platinum drugs. Molecular Oncology 2:94–101. doi:10.1016/j.molonc.2008.01.003
Isayev O et al. (2014) Inhibition of glucose turnover by 3-bromopyruvate counteracts pancreatic cancer stem cell features and sensitizes cells to gemcitabine. Oncotarget 5:5177–5189
Jardim-Messeder D, Moreira-Pacheco F (2016) 3-Bromopyruvic Acid Inhibits Tricarboxylic Acid Cycle and Glutaminolysis in HepG2 Cells. Anticancer Res 36(5):2233–2241
Kim W et al. (2007) Apoptosis-inducing antitumor efficacy of hexokinase II inhibitor in hepatocellular carcinoma. Mol Cancer Ther 6:2554–2562. doi:10.1158/1535-7163.MCT-07-0115
Kim JS et al. (2008) Role of reactive oxygen species-mediated mitochondrial dysregulation in 3-bromopyruvate induced cell death in hepatoma cells : ROS-mediated cell death by 3-BrPA. J Bioenerg Biomembr 40:607–618. doi:10.1007/s10863-008-9188-0
Ko YH, Pedersen PL, Geschwind JF (2001) Glucose catabolism in the rabbit VX2 tumor model for liver cancer: characterization and targeting hexokinase. Cancer Lett 173:83–91
Ko YH et al. (2004) Advanced cancers: eradication in all cases using 3-bromopyruvate therapy to deplete ATP. Biochem Biophys Res Commun 324:269–275. doi:10.1016/j.bbrc.2004.09.047
Ko YH, Verhoeven HA, Lee MJ, Corbin DJ, Vogl TJ, Pedersen PL (2012) A translational study "case report" on the small molecule "energy blocker" 3-bromopyruvate (3BP) as a potent anticancer agent: from bench side to bedside. J Bioenerg Biomembr 44:163–170. doi:10.1007/s10863-012-9417-4
Kunjithapatham R, Geschwind JF, Rao PP, Boronina TN, Cole RN, Ganapathy-Kanniappan S (2013) Systemic administration of 3-bromopyruvate reveals its interaction with serum proteins in a rat model. BMC Res Notes 6:277. doi:10.1186/1756-0500-6-277
Liu XH, Zheng XF, Wang YL (2009) Inhibitive effect of 3-bromopyruvic acid on human breast cancer MCF-7 cells involves cell cycle arrest and apoptotic induction. Chin Med J (Engl) 122:1681–1685
Liu Z, Zhang YY, Zhang QW, Zhao SR, CZ W, Cheng X, Jiang CC, Jiang ZW, Liu H (2014) 3-Bromopyruvate induces apoptosis in breast cancer cells by downregulating Mcl-1 through the PI3K/Akt signaling pathway. Anti-Cancer Drugs 25(4):447–455. doi:10.1097/CAD.0000000000000081
Macchioni L et al. (2011) Mitochondrial dysfunction and effect of antiglycolytic bromopyruvic acid in GL15 glioblastoma cells J Bioenerg Biomembr 43:507–518 doi:10.1007/s10863-011-9375-2
Macchioni L, Davidescu M, Roberti R, Corazzi L (2014) The energy blockers 3-bromopyruvate and lonidamine: effects on bioenergetics of brain mitochondria. J Bioenerg Biomembr 46(5):389–394. doi:10.1007/s10863-014-9577-5
Madhukar NS, Warmoes MO, Locasale JW (2015) Organization of enzyme concentration across the metabolic network in cancer cells. PLoS One 10:e0117131. doi:10.1371/journal.pone.0117131
Majkowska-Skrobek G et al. (2014) Killing multiple myeloma cells with the small molecule 3-bromopyruvate: implications for therapy. Anti-cancer Drugs 25:673–682. doi:10.1097/CAD.0000000000000094
Marrache S, Dhar S (2015) The energy blocker inside the power house: Mitochondria targeted delivery of 3-bromopyruvate. Chem Sci 6(3):1832–1845. doi:10.1039/c4sc01963f
Mathupala SP, Ko YH, Pedersen PL (2006) Hexokinase II: cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene 25:4777–4786. doi:10.1038/sj.onc.1209603
Mathupala SP, Ko YH, Pedersen PL (2009) Hexokinase-2 bound to mitochondria: cancer’s stygian link to the "Warburg Effect" and a pivotal target for effective therapy. Semin Cancer Biol 19(1):17–24. doi:10.1016/j.semcancer.2008.11.006
Matsumoto S et al. (2013) EPR oxygen imaging and hyperpolarized 13C MRI of pyruvate metabolism as noninvasive biomarkers of tumor treatment response to a glycolysis inhibitor 3-bromopyruvate. Magn Reson Med 69:1443–1450. doi:10.1002/mrm.24355
Matsushita K et al. (2012) Glycolysis inhibitors as a potential therapeutic option to treat aggressive neuroblastoma expressing GLUT1. J Pediatr Surg 47:1323–1330. doi:10.1016/j.jpedsurg.2011.12.007
Meloche HP (1967) Bromopyruvate inactivation of 2-keto-3-deoxy-6-phosphogluconic aldolase. I. Kinetic evidence for active site specificity. Biochemistry 6:2273–2280
Michelakis ED et al. (2010) Metabolic modulation of glioblastoma with dichloroacetate Sci Transl Med 2:31ra34 doi:10.1126/scitranslmed.3000677
Nakano A et al. (2011) Glycolysis inhibition inactivates ABC transporters to restore drug sensitivity in malignant cells. PLoS One 6:e27222. doi:10.1371/journal.pone.0027222
Nakano A et al. (2012) Up-regulation of hexokinaseII in myeloma cells: targeting myeloma cells with 3-bromopyruvate. J Bioenerg Biomembr 44:31–38. doi:10.1007/s10863-012-9412-9
Nilsson H, Lindgren D, Mandahl Forsberg A, Mulder H, Axelson H, Johansson ME (2015) Primary clear cell renal carcinoma cells display minimal mitochondrial respiratory capacity resulting in pronounced sensitivity to glycolytic inhibition by 3-Bromopyruvate. Cell Death Dis 6:e1585. doi:10.1038/cddis.2014.545
Oronsky BT, Reid T, Knox SJ, Scicinski JJ (2012) The scarlet letter of alkylation: a mini review of selective alkylating agents. Transl Oncol 5:226–229
Ota S, Geschwind JF, Buijs M, Wijlemans JW, Kwak BK, Ganapathy-Kanniappan S (2013) Ultrasound-guided direct delivery of 3-bromopyruvate blocks tumor progression in an orthotopic mouse model of human pancreatic cancer. Target Oncol 8:145–151. doi:10.1007/s11523-013-0273-x
Parks SK, Chiche J, Pouyssegur J (2013) Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer 13:611–623. doi:10.1038/nrc3579
Pedersen PL (2007) Warburg, me and Hexokinase 2: Multiple discoveries of key molecular events underlying one of cancers’ most common phenotypes, the "Warburg Effect", i.e., Elevated glycolysis in the presence of oxygen. J Bioenerg Biomembr 39(3):211–222
Pedersen PL (2012a) 3-bromopyruvate (3BP) a fast acting, promising, powerful, specific, and effective "small molecule" anti-cancer agent taken from labside to bedside: introduction to a special issue. J Bioenerg Biomembr 44(1):1–6. doi:10.1007/s10863-012-9425-4
Pedersen PL (2012b) Mitochondria in relation to cancer metastasis: introduction to a mini-review series. J Bioenerg Biomembr 44(6):615–617. doi:10.1007/s10863-012-9470-z
Pereira da Silva AP et al. (2009) Inhibition of energy-producing pathways of HepG2 cells by 3-bromopyruvate. Biochem J 417:717–726. doi:10.1042/BJ20080805
Pinheiro C, Longatto-Filho A, Azevedo-Silva J, Casal M, Schmitt FC, Baltazar F (2012) Role of monocarboxylate transporters in human cancers: state of the art. J Bioenerg Biomembr 44:127–139. doi:10.1007/s10863-012-9428-1
Qin JZ, Xin H, Nickoloff BJ (2010) 3-Bromopyruvate induces necrotic cell death in sensitive melanoma cell lines. Biochem Biophys Res Commun 396:495–500. doi:10.1016/j.bbrc.2010.04.126
Queiros O et al. (2012) Butyrate activates the monocarboxylate transporter MCT4 expression in breast cancer cells and enhances the antitumor activity of 3-bromopyruvate. J Bioenerg Biomembr 44:141–153. doi:10.1007/s10863-012-9418-3
Rodrigues-Ferreira C, da Silva AP, Galina A (2012) Effect of the antitumoral alkylating agent 3-bromopyruvate on mitochondrial respiration: role of mitochondrially bound hexokinase. J Bioenerg Biomembr 44:39–49. doi:10.1007/s10863-012-9413-8
Sadowska-Bartosz I, Bartosz G (2013) Effect of 3-bromopyruvic acid on human erythrocyte antioxidant defense system. Cell Biol Int 37:1285–1290. doi:10.1002/cbin.10160
Sadowska-Bartosz I, Soszynski M, Ulaszewski S, Ko Y, Bartosz G (2014) Transport of 3-bromopyruvate across the human erythrocyte membrane. Cell Mol Biol Lett 19:201–214. doi:10.2478/s11658-014-0189-1
Sakamoto H, Mashima T, Kizaki A, Dan S, Hashimoto Y, Naito M, Tsuruo T (2000) Glyoxalase I is involved in resistance of human leukemia cells to antitumor agent-induced apoptosis. Blood 95:3214–3218
Sanchez-Arago M, Cuezva JM (2011) The bioenergetic signature of isogenic colon cancer cells predicts the cell death response to treatment with 3-bromopyruvate, iodoacetate or 5-fluorouracil. J Transl Med 9(19). doi:10.1186/1479-5876-9-19
Schaefer NG, Geschwind JF, Engles J, Buchanan JW, Wahl RL (2012) Systemic administration of 3-bromopyruvate in treating disseminated aggressive lymphoma. Transl Res 159:51–57. doi:10.1016/j.trsl.2011.08.008
Shoshan MC (2012) 3-bromopyruvate: targets and outcomes. J Bioenerg Biomembr 44:7–15. doi:10.1007/s10863-012-9419-2
Sonveaux P et al. (2008) Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest 118(12):3930–3942. doi:10.1172/JCI36843
Staub M, Denes G (1967) A kinetic study on the inactivation of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase by bromopyruvate. Biochim Biophys Acta 139:519–521
Szablewski L (2013) Expression of glucose transporters in cancers. Biochim Biophys Acta 1835:164–169. doi:10.1016/j.bbcan.2012.12.004
Tang Z et al. (2012) Over-expression of GAPDH in human colorectal carcinoma as a preferred target of 3-bromopyruvate propyl ester. J Bioenerg Biomembr 44:117–125. doi:10.1007/s10863-012-9420-9
Thangaraju M, Karunakaran SK, Itagaki S, Gopal E, Elangovan S, Prasad PD, Ganapathy V (2009) Transport by SLC5A8 with subsequent inhibition of histone deacetylase 1 (HDAC1) and HDAC3 underlies the antitumor activity of 3-bromopyruvate. Cancer 115:4655–4666. doi:10.1002/cncr.24532
Thornalley PJ, Rabbani N (2011) Glyoxalase in tumourigenesis and multidrug resistance. Sem Cell Dev Biol 22:318–325. doi:10.1016/j.semcdb.2011.02.006
Tristan C, Shahani N, Sedlak TW, Sawa A (2011) The diverse functions of GAPDH: views from different subcellular compartments. Cell Signal 23:317–323. doi:10.1016/j.cellsig.2010.08.003
Tsai HJ, Wilson JE (1996) Functional organization of mammalian hexokinases: both N- and C-terminal halves of the rat type II isozyme possess catalytic sites. Arch Biochem Biophys 329:17–23. doi:10.1006/abbi.1996.0186
Valenti D, Vacca RA, de Bari L (2015) 3-Bromopyruvate induces rapid human prostate cancer cell death by affecting cell energy metabolism, GSH pool and the glyoxalase system. J Bioenerg Biomembr 47:493–506 doi:10.1997/s10863-015-9631-y
Vali M et al. (2007) Intraarterial therapy with a new potent inhibitor of tumor metabolism (3-bromopyruvate): identification of therapeutic dose and method of injection in an animal model of liver cancer. J Vasc Interv Radiol 18:95–101. doi:10.1016/j.jvir.2006.10.019
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–1033. doi:10.1126/science.1160809
Verhoeven HA, van Griensven LJ (2012) Flow cytometric evaluation of the effects of 3-bromopyruvate (3BP) and dichloracetate (DCA) on THP-1 cells: a multiparameter analysis. J Bioenerg Biomembr 44:91–99. doi:10.1007/s10863-012-9414-7
Vossen JA, Buijs M, Syed L, Kutiyanwala F, Kutiyanwala M, Geschwind JF, Vali M (2008) Development of a new orthotopic animal model of metastatic liver cancer in the rabbit VX2 model: effect on metastases after partial hepatectomy, intra-arterial treatment with 3-bromopyruvate and chemoembolization. Clin Exp metastasis 25:811–817. doi:10.1007/s10585-008-9195-x
Warburg O (1956) On respiratory impairment in cancer cells, vol 124. Science, pp. 269–270
Wicks RT et al. (2015) Local delivery of cancer-cell glycolytic inhibitors in high-grade glioma. Neuro-Oncology 17:70–80. doi:10.1093/neuonc/nou143
Wintzell M, Lofstedt L, Johansson J, Pedersen AB, Fuxe J, Shoshan M (2012) Repeated cisplatin treatment can lead to a multiresistant tumor cell population with stem cell features and sensitivity to 3-bromopyruvate. Cancer Biol Ther 13:1454–1462. doi:10.4161/cbt.22007
Wu L et al. (2014) The reversal effects of 3-bromopyruvate on multidrug resistance in vitro and in vivo derived from human breast MCF-7/ADR cells. PLoS One 9:e112132 doi:10.1371/journal.pone.0112132
Xian SL, Cao W, Zhang XD, Lu YF (2014) Inhibitory effects of 3-bromopyruvate on human gastric cancer implant tumors in nude mice Asian Pac. J Cancer Prev 15:3175–3178
Xiao H, Li S, Zhang D, Liu T, Yu M, Wang F (2013) Separate and concurrent use of 2-deoxy-D-glucose and 3-bromopyruvate in pancreatic cancer cells. Oncol Rep 29:329–334. doi:10.3892/or.2012.2085
Xu RH, Pelicano H, Zhang H, Giles FJ, Keating MJ, Huang P (2005) Synergistic effect of targeting mTOR by rapamycin and depleting ATP by inhibition of glycolysis in lymphoma and leukemia cells. Leukemia 19:2153–2158. doi:10.1038/sj.leu.2403968
Yoong SL et al. (2014) Enhanced cytotoxicity to cancer cells by mitochondria-targeting MWCNTs containing platinum(IV) prodrug of cisplatin. Biomaterials 35:748–759. doi:10.1016/j.biomaterials.2013.09.036
Yu SJ et al. (2011) Inhibition of hypoxia-inducible carbonic anhydrase-IX enhances hexokinase II inhibitor-induced hepatocellular carcinoma cell apoptosis. Acta Pharmacol Sin 32:912–920. doi:10.1038/aps.2011.24
Yu SJ et al. (2012) Enhancement of hexokinase II inhibitor-induced apoptosis in hepatocellular carcinoma cells via augmenting ER stress and anti-angiogenesis by protein disulfide isomerase inhibition. J Bioenerg Biomembr 44:101–115. doi:10.1007/s10863-012-9416-5
Yun J et al. (2009) Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science 325:1555–1559. doi:10.1126/science.1174229
Zhang Q, Pan J, North PE, Yang S, Lubet RA, Wang Y, You M (2012) Aerosolized 3-bromopyruvate inhibits lung tumorigenesis without causing liver toxicity. Cancer Prev Res (Phila) 5:717–725. doi:10.1158/1940-6207.CAPR-11-0338
Zhang Q et al. (2014) Hexokinase II inhibitor, 3-BrPA induced autophagy by stimulating ROS formation in human breast cancer cells. Genes Cancer 5:100–112
Zhou Y et al. (2012) Intracellular ATP levels are a pivotal determinant of chemoresistance in colon cancer cells. Cancer Res 72:304–314. doi:10.1158/0008-5472.CAN-11-1674
Acknowledgments
This work was supported by the strategic programme UID/BIA/04050/2013 (POCI-01-0145-FEDER-007569) funded by national funds through the FCT I.P. and by the ERDF through the COMPETE2020 - Programa Operacional Competitividade e Internacionalização (POCI). João Azevedo-Silva received a fellowship from the Portuguese government from the FCT through FSE (Fundo Social Europeu) and POPH (Programa Operacional Potencial Humano) [grant number SFRH/BD/76038/2011]. Co-author Peter L. Pedersen was supported by NIH grant NCI CA l0951 for many years for cancer research that led to a number of the findings described in this review.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Azevedo-Silva, J., Queirós, O., Baltazar, F. et al. The anticancer agent 3-bromopyruvate: a simple but powerful molecule taken from the lab to the bedside. J Bioenerg Biomembr 48, 349–362 (2016). https://doi.org/10.1007/s10863-016-9670-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-016-9670-z