Abstract
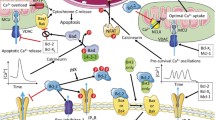
Bcl-2 family proteins, known for their apoptosis functioning at the mitochondria, have been shown to localize to other cellular compartments to mediate calcium (Ca2+) signals. Since the proper supply of Ca2+ in cells serves as an important mechanism for cellular survival and bioenergetics, we propose an integrating role for Bcl-2 family proteins in modulating Ca2+ signaling. The endoplasmic reticulum (ER) is the main Ca2+ storage for the cell and Bcl-2 family proteins competitively regulate its Ca2+ concentration. Bcl-2 family proteins also regulate the flux of Ca2+ from the ER by physically interacting with inositol 1,4,5-trisphosphate receptors (IP3Rs) to mediate their opening. Type 1 IP3Rs reside at the bulk ER to coordinate cytosolic Ca2+ signals, while type 3 IP3Rs reside at mitochondria-associated ER membrane (MAM) to facilitate mitochondrial Ca2+ uptake. In healthy cells, mitochondrial Ca2+ drives pyruvate into the citric acid (TCA) cycle to facilitate ATP production, while a continuous accumulation of Ca2+ can trigger the release of cytochrome c, thus initiating apoptosis. Since multiple organelles and Bcl-2 family proteins are involved in Ca2+ signaling, we aim to clarify the role that Bcl-2 family proteins play in facilitating Ca2+ signaling and how mitochondrial Ca2+ is relevant in both bioenergetics and apoptosis. We also explore how these insights could be useful in controlling bioenergetics in apoptosis-resistant cell lines.
Similar content being viewed by others
References
Arnaudeau S, Frieden M, Nakamura K et al (2002) Calreticulin differentially modulates calcium uptake and release in the endoplasmic reticulum and mitochondria. J Biol Chem 277:46696–46705
Baffy G, Miyashita T, Williamson JR, Reed JC (1993) Apoptosis induced by withdrawal of interleukin-3 (IL-3) from an IL-3-dependent hematopoietic cell line is associated with repartitioning of intracellular calcium and is blocked by enforced Bcl-2 oncoprotein production. J Biol Chem 268:6511–6519
Baines CP, Kaiser RA, Sheiko T et al (2007) Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol 9:550–555
Baughman JM, Perocchi F, Girgis HS et al (2011) Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476:341–345
Boehning D, Patterson RL, Sedaghat L et al (2003) Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol 5:1051–1061
Brunelle JK, Letai A (2009) Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci 122:437–441
Burlacu A (2003) Regulation of apoptosis by Bcl-2 family proteins. J Cell Mol Med 7:249–257
Chaudhuri D, Sancak Y, Mootha VK, Clapham DE (2013) MCU encodes the pore conducting mitochondrial calcium currents. Elife 2:e00704
Chen M, Won D-J, Krajewski S, Gottlieb RA (2002) Calpain and mitochondria in ischemia/reperfusion injury. J Biol Chem 277:29181–29186
Chen R, Valencia I, Zhong F et al (2004) Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J Cell Biol 166:193–203
Chen Y, Lewis W, Diwan A et al (2010) Dual autonomous mitochondrial cell death pathways are activated by Nix/BNip3L and induce cardiomyopathy. Proc Natl Acad Sci U S A 107:9035–9042
Cheng EH, Wei MC, Weiler S et al (2001) BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell 8:705–711
Chiang GG, Sisk WP (2005) Bcl-x(L) mediates increased production of humanized monoclonal antibodies in Chinese hamster ovary cells. Biotechnol Bioeng 91:779–792
Chou C, Lee R, Yang-Yen H-F (2006) An internal EELD domain facilitates mitochondrial targeting of Mcl-1 via a Tom70-dependent pathway. Mol Biol Cell 17:3952–3963
Cost GJ, Freyvert Y, Vafiadis A et al (2010) BAK and BAX deletion using zinc-finger nucleases yields apoptosis-resistant CHO cells. Biotechnol Bioeng 105:330–340
Csordás G, Renken C, Várnai P et al (2006) Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol 174:915–921
Dejean LM, Martinez-Caballero S, Guo L et al (2005) Oligomeric Bax is a component of the putative cytochrome c release channel MAC, mitochondrial apoptosis-induced channel. Mol Biol Cell 16:2424–2432
Dejean LM, Martinez-Caballero S, Manon S, Kinnally KW (2006) Regulation of the mitochondrial apoptosis-induced channel, MAC, by BCL-2 family proteins. Biochim Biophys Acta 1762:191–201
Diwan A, Matkovich SJ, Yuan Q et al (2009) Endoplasmic reticulum-mitochondria crosstalk in NIX-mediated murine cell death. J Clin Invest 119:203–212
Dorai H, Kyung YS, Ellis D et al (2009) Expression of anti-apoptosis genes alters lactate metabolism of Chinese Hamster Ovary cells in culture. Biotechnol Bioeng 103:592–608
Eckenrode EF, Yang J, Velmurugan GV et al (2010) Apoptosis protection by Mcl-1 and Bcl-2 modulation of inositol 1,4,5-trisphosphate receptor-dependent Ca2+ signaling. J Biol Chem 285:13678–13684
Edlich F, Banerjee S, Suzuki M et al (2011) Bcl-x(L) retrotranslocates Bax from the mitochondria into the cytosol. Cell 145:104–116
Erickson ES, Mooren OL, Moore D et al (2006) The role of nuclear envelope calcium in modifying nuclear pore complex structure. Can J Physiol Pharm 84:309–318
Gallenne T, Gautier F, Oliver L et al (2009) Bax activation by the BH3-only protein Puma promotes cell dependence on antiapoptotic Bcl-2 family members. J Cell Biol 185:279–290
Gélinas C, White E (2005) BH3-only proteins in control: specificity regulates MCL-1 and BAK-mediated apoptosis. Gene Dev 19:1263–1268
George NM, Targy N, Evans JJD et al (2010) Bax contains two functional mitochondrial targeting sequences and translocates to mitochondria in a conformational change- and homo-oligomerization-driven process. J Biol Chem 285:1384–1392
Gerace L, Ottaviano Y, Kondor-Koch C (1982) Identification of a major polypeptide of the nuclear pore complex. J Cell Biol 95:826–837
Germain M, Duronio V (2007) The N terminus of the anti-apoptotic BCL-2 homologue MCL-1 regulates its localization and function. J Biol Chem 282:32233–32242
Gil-Parrado S, Fernández-Montalván A, Assfalg-Machleidt I et al (2002) Ionomycin-activated calpain triggers apoptosis. A probable role for Bcl-2 family members. J Biol Chem 277:27217–27226
Giorgio V, von Stockum S, Antoniel M et al (2013) Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci U S A 110:5887–5892
Görlach A, Klappa P, Kietzmann T (2006) The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Sign 8:1391–1418
Greber UF, Gerace L (1995) Depletion of calcium from the lumen of endoplasmic reticulum reversibly inhibits passive diffusion and signal-mediated transport into the nucleus. J Cell Biol 128:5–14
Gunter TE, Sheu S-S (2009) Characteristics and possible functions of mitochondrial Ca(2+) transport mechanisms. Biochim Biophys Acta 1787:1291–1308
Guo L, Pietkiewicz D, Pavlov EV et al (2004) Effects of cytochrome c on the mitochondrial apoptosis-induced channel MAC. Am J Physiol Cell Physiol 286:C1109–C1117
Hajnóczky G, Csordás G, Yi M (2002) Old players in a new role: mitochondria-associated membranes, VDAC, and ryanodine receptors as contributors to calcium signal propagation from endoplasmic reticulum to the mitochondria. Cell Calcium 32:363–377
Hajnóczky G, Roy SS, Madesh M et al (2009) Bad targets the permeability transition pore independent of Bax or Bak to switch between Ca2+− dependent cell survival and death. Mol Cell 33:377–388
Hanson CJ, Bootman MD, Distelhorst CW et al (2008) The cellular concentration of Bcl-2 determines its pro- or anti-apoptotic effect. Cell Calcium 44:243–258
Hayashi T, Su T-P (2007) Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131:596–610
Hayashi T, Su T-P, Rizzuto R, Hajnoczky G (2009) MAM: more than just a housekeeper. Trends Cell Biol 19:81–88
He H, McCormick TS, Lam M, Distelhorst CW (1997) Maintenance of calcium homeostasis in the endoplasmic reticulum by Bcl-2. J Cell Biol 138:1219–1228
Huang X, Zhai D, Huang Y (2000) Study on the relationship between calcium-induced calcium release from mitochondria and PTP opening. Mol Cell Biochem 213:29–35
Itoh Y, Ueda H, Suzuki E (1995) Overexpression of bcl-2, apoptosis suppressing gene: Prolonged viable culture period of hybridoma and enhanced antibody production. Biotechnol Bioeng 48:118–122
Jeong S-Y, Seol D-W (2008) The role of mitochondria in apoptosis. BMB Rep 41:11–22
Jeong S-Y, Gaume B, Lee Y-J et al (2004) Bcl-x(L) sequesters its C-terminal membrane anchor in soluble, cytosolic homodimers. EMBO J 23:2146–2155
John LM, Lechleiter JD, Camacho P (1998) Differential modulation of SERCA2 isoforms by calreticulin. J Cell Biol 142:963–973
Jouaville LS, Ichas F, Mazat JP (1998) Modulation of cell calcium signals by mitochondria. Mol Cell Biochem 184:371–376
Kaufmann T, Schlipf S, Sanz J et al (2003) Characterization of the signal that directs Bcl-xL, but not Bcl-2, to the mitochondrial outer membrane. J Cell Biol 160:53–64
Khorchid A, Ikura M (2002) How calpain is activated by calcium. Nat Struct Biol 9:239–241
Kokoszka JE, Waymire KG, Levy SE et al (2004) The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 427:461–465
Korsmeyer SJ, Wei MC, Saito M et al (2000) Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ 7:1166–1173
Li C, Fox CJ, Master SR et al (2002) Bcl-X(L) affects Ca(2+) homeostasis by altering expression of inositol 1,4,5-trisphosphate receptors. Proc Natl Acad Sci U S A 99:9830–9835
Li C, Wang X, Vais H et al (2007) Apoptosis regulation by Bcl-x(L) modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proc Natl Acad Sci U S A 104:12565–12570
Lindsay J, Esposti MD, Gilmore AP (2011) Bcl-2 proteins and mitochondria–specificity in membrane targeting for death. Biochim Biophys Acta 1813:532–539
Łopatniuk P, Witkowski JM (2011) Conventional calpains and programmed cell death. Acta Biochim Pol 58:287–296
Luo X, He Q, Huang Y, Sheikh MS (2005) Transcriptional upregulation of PUMA modulates endoplasmic reticulum calcium pool depletion-induced apoptosis via Bax activation. Cell Death Differ 12:1310–1318
Majors BS, Betenbaugh MJ, Chiang GG (2007) Links between metabolism and apoptosis in mammalian cells: applications for anti-apoptosis engineering. Metab Eng 9:317–326
Majors BS, Betenbaugh MJ, Pederson NE, Chiang GG (2009) Mcl-1 overexpression leads to higher viabilities and increased production of humanized monoclonal antibody in Chinese hamster ovary cells. Biotechnol Progr 25:1161–1168
Mannella CA, Buttle K, Rath BK, Marko M (1998) Electron microscopic tomography of rat-liver mitochondria and their interactions with the endoplasmic reticulum. BioFactors 8:225–228
Martínez VS, Dietmair S, Quek L-E et al (2013) Flux balance analysis of CHO cells before and after a metabolic switch from lactate production to consumption. Biotechnol Bioeng 110:660–666
Martinez-Caballero S, Dejean LM, Kinnally KW (2004) Some amphiphilic cations block the mitochondrial apoptosis-induced channel, MAC. FEBS Lett 568:35–38
Martinez-Caballero S, Dejean LM, Jonas EA, Kinnally KW (2005) The role of the mitochondrial apoptosis induced channel MAC in cytochrome c release. J Bioenerg Biomembr 37:155–164
Masters SC, Yang H, Datta SR et al (2001) 14-3-3 inhibits Bad-induced cell death through interaction with serine-136. Mol Pharmacol 60:1325–1331
Mastrangelo AJ, Zou S, Hardwick JM, Betenbaugh MJ (1999) Antiapoptosis chemicals prolong productive lifetimes of mammalian cells upon Sindbis virus vector infection. Biotechnol Bioeng 65:298–305
Mastrangelo AJ, Hardwick JM, Bex F, Betenbaugh MJ (2000) Part I. Bcl-2 and Bcl-x(L) limit apoptosis upon infection with alphavirus vectors. Biotechnol Bioeng 67:544–554
Mathai JP, Germain M, Marcellus RC, Shore GC (2002) Induction and endoplasmic reticulum location of BIK/NBK in response to apoptotic signaling by E1A and p53. Oncogene 21:2534–2544
Mathai JP, Germain M, Shore GC (2005) BH3-only BIK regulates BAX, BAK-dependent release of Ca2+ from endoplasmic reticulum stores and mitochondrial apoptosis during stress-induced cell death. J Biol Chem 280:23829–23836
Mendes CCP, Gomes DA, Thompson M et al (2005) The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria. J Biol Chem 280:40892–40900
Meunier J, Hayashi T (2010) Sigma-1 receptors regulate Bcl-2 expression by reactive oxygen species-dependent transcriptional regulation of nuclear factor kappaB. J Pharmacol Exp Ther 332:388–397
Minagawa N, Kruglov EA, Dranoff JA et al (2005) The anti-apoptotic protein Mcl-1 inhibits mitochondrial Ca2+ signals. J Biol Chem 280:33637–33644
Murphy RC, Schneider E, Kinnally KW (2001) Overexpression of Bcl-2 suppresses the calcium activation of a mitochondrial megachannel. FEBS Lett 497:73–76
Nakagawa T, Shimizu S, Watanabe T et al (2005) Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434:652–658
Nakano K, Vousden KH (2001) PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell 7:683–694
Nijhawan D, Fang M, Traer E et al (2003) Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Gene Dev 17:1475–1486
Nivitchanyong T, Martinez A, Ishaque A et al (2007) Anti-apoptotic genes Aven and E1B-19K enhance performance of BHK cells engineered to express recombinant factor VIII in batch and low perfusion cell culture. Biotechnol Bioeng 98:825–841
Oakes SA, Scorrano L, Opferman JT et al (2005) Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci U S A 102:105–110
Papas KK, Sun L, Roos ES et al (1999) Change in lactate production in Myc-transformed cells precedes apoptosis and can be inhibited by Bcl-2 overexpression. FEBS Lett 446:338–342
Petch A, Al-Rubeai M (2004) The Bcl-2 family. In: Al-Rubeai M, Fussenegger M (eds) Cell engineering. Kluwer Academic Publishers, Netherlands, pp 25–47
Pinton P (2000) Reduced loading of intracellular Ca2+ stores and downregulation of capacitative Ca2+ influx in Bcl-2-overexpressing cells. J Cell Biol 148:857–862
Pinton P, Ferrari D, Rapizzi E et al (2002) A role for calcium in Bcl-2 action? Biochimie 84:195–201
Portier BP, Taglialatela G (2006) Bcl-2 localized at the nuclear compartment induces apoptosis after transient overexpression. J Biol Chem 281:40493–40502
Puthalakath H, Huang DC, O’Reilly LA et al (1999) The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell 3:287–296
Putney JW (2005) Capacitative calcium entry: sensing the calcium stores. J Cell Biol 169:381–382
Rapizzi E, Pinton P, Szabadkai G et al (2002) Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J Cell Biol 159:613–624
Reimertz C, Kögel D, Rami A et al (2003) Gene expression during ER stress-induced apoptosis in neurons: induction of the BH3-only protein Bbc3/PUMA and activation of the mitochondrial apoptosis pathway. J Cell Biol 162:587–597
Rizzuto R, De Stefani D, Marchi S et al (2009) Ca(2+) transfer from the ER to mitochondria: when, how and why. Biochim Biophys Acta 1787:1342–1351
Roderick HL, Lechleiter JD, Camacho P (2000) Cytosolic phosphorylation of calnexin controls intracellular Ca(2+) oscillations via an interaction with SERCA2b. J Cell Biol 149:1235–1248
Rong Y-P, De Smedt H, Bultynck G et al (2009) The BH4 domain of Bcl-2 inhibits ER calcium release and apoptosis by binding the regulatory and coupling domain of the IP3 receptor. Proc Natl Acad Sci U S A 106:14397–14402
Rostovtseva TK, Antonsson B, Suzuki M et al (2004) Bid, but not Bax, regulates VDAC channels. J Biol Chem 279:13575–13583
Ruffolo SC, Shore GC (2003) BCL-2 selectively interacts with the BID-induced open conformer of BAK, inhibiting BAK auto-oligomerization. J Biol Chem 278:25039–25045
Salido GM, Sage SO, Rosado JA (2009) Biochemical and functional properties of the store-operated Ca2+ channels. Cell Signal 21:457–461
Scorrano L, Oakes SA, Opferman JT et al (2003) BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science 300:135–139
Shimizu S, Ide T, Yanagida T, Tsujimoto Y (2000a) Electrophysiological study of a novel large pore formed by Bax and the voltage-dependent anion channel that is permeable to cytochrome c. J Biol Chem 275:12321–12325
Shimizu S, Konishi A, Kodama T, Tsujimoto Y (2000b) BH4 domain of antiapoptotic Bcl-2 family members closes voltage-dependent anion channel and inhibits apoptotic mitochondrial changes and cell death. Proc Natl Acad Sci U S A 97:3100–3105
Shore GC, Nguyen M (2008) Bcl-2 proteins and apoptosis: choose your partner. Cell 135:1004–1006
Shore GC, Tata JR (1977) Two fractions of rough endoplasmic reticulum from rat liver. II. Cytoplasmic messenger RNA’s which code for albumin and mitochondrial proteins are distributed differently between the two fractions. J Cell Biol 72:726–743
Spät A, Szanda G, Csordás G, Hajnóczky G (2008) High- and low-calcium-dependent mechanisms of mitochondrial calcium signalling. Cell Calcium 44:51–63
Springer JE, Azbill RD, Nottingham SA, Kennedy SE (2000) Calcineurin-mediated BAD dephosphorylation activates the caspase-3 apoptotic cascade in traumatic spinal cord injury. J Neurosci 20:7246–7251
Strasser C, Grote P, Schäuble K et al (2012) Regulation of nuclear envelope permeability in cell death and survival. Nucleus 3:540–551
Szabadkai G, Bianchi K, Várnai P et al (2006) Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol 175:901–911
Tagami S, Eguchi Y, Kinoshita M et al (2000) A novel protein, RTN-XS, interacts with both Bcl-XL and Bcl-2 on endoplasmic reticulum and reduces their anti-apoptotic activity. Oncogene 19:5736–5746
Tan W, Colombini M (2007) VDAC closure increases calcium ion flux. Biochim Biophys Acta 1768:2510–2515
Tsujimoto Y, Shimizu S (2000) VDAC regulation by the Bcl-2 family of proteins. Cell Death Differ 7:1174–1181
Vafiadaki E, Arvanitis DA, Pagakis SN et al (2009) The Anti-apoptotic Protein HAX-1 Interacts with SERCA2 and Regulates Its Protein Levels to Promote Cell Survival. Mol Biol Cell 20:306–318
Vance JE (2003) Molecular and cell biology of phosphatidylserine and phosphatidylethanolamine metabolism. Prog Nucleic Acid Re 75:69–111
Vanden Abeele F, Skryma R, Shuba Y et al (2002) Bcl-2-dependent modulation of Ca(2+) homeostasis and store-operated channels in prostate cancer cells. Cancer Cell 1:169–179
Vander Heiden MG, Thompson CB (1999) Bcl-2 proteins: regulators of apoptosis or of mitochondrial homeostasis? Nat Cell Biol 1:E209–E216
Vander Heiden MG, Chandel NS, Li XX et al (2000) Outer mitochondrial membrane permeability can regulate coupled respiration and cell survival. Proc Natl Acad Sci U S A 97:4666–4671
Vander Heiden MG, Li XX, Gottleib E et al (2001) Bcl-xL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane. J Biol Chem 276:19414–19419
Walter L, Hajnóczky G (2005) Mitochondria and endoplasmic reticulum: the lethal interorganelle cross-talk. J Bioenerg Biomembr 37:191–206
Wang H (1999) Ca2+− induced apoptosis through calcineurin dephosphorylation of BAD. Science 284:339–343
Wang NS, Unkila MT, Reineks EZ, Distelhorst CW (2001) Transient expression of wild-type or mitochondrially targeted Bcl-2 induces apoptosis, whereas transient expression of endoplasmic reticulum-targeted Bcl-2 is protective against Bax-induced cell death. J Biol Chem 276:44117–44128
White C, Li C, Yang J et al (2005) The endoplasmic reticulum gateway to apoptosis by Bcl-XL modulation of the InsP3R. Nat Cell Biol 7:1021–1028
Williams SS, French JN, Gilbert M et al (2000) Bcl-2 overexpression results in enhanced capacitative calcium entry and resistance to SKF-96395-induced apoptosis. Cancer Res 60:4358–4361
Willis SN, Chen L, Dewson G et al (2005) Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Gene Dev 19:1294–1305
Yap SV, Vafiadaki E, Strong J, Kontrogianni-Konstantopoulos A (2010) HAX-1: a multifaceted antiapoptotic protein localizing in the mitochondria and the sarcoplasmic reticulum of striated muscle cells. J Mol Cell Cardiol 48:1266–1279
Yi X, Yin X-M, Dong Z (2003) Inhibition of Bid-induced apoptosis by Bcl-2. tBid insertion, Bax translocation, and Bax/Bak oligomerization suppressed. J Biol Chem 278:16992–16999
Zannetti A, Iommelli F, Fonti R et al (2008) Gefitinib induction of in vivo detectable signals by Bcl-2/Bcl-xL modulation of inositol trisphosphate receptor type 3. Clin Cancer Res 14:5209–5219
Zhang L, Li L, Liu H et al (2009) BNIP3 mediates cell death by different pathways following localization to endoplasmic reticulum and mitochondrion. FASEB J 23:3405–3414
Zhang S, Fritz N, Ibarra C, Uhlén P (2011) Inositol 1,4,5-trisphosphate receptor subtype-specific regulation of calcium oscillations. Neurochem Res 36:1175–1185
Zhao X, Wang L, Sun Y et al (2008) The endoplasmic reticulum (ER)-target protein Bik induces Hep3B cells apoptosis by the depletion of the ER Ca2+ stores. Mol Cell Biochem 312:33–38
Zong W-X, Li C, Hatzivassiliou G et al (2003) Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J Cell Biol 162:59–69
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lewis, A., Hayashi, T., Su, TP. et al. Bcl-2 family in inter-organelle modulation of calcium signaling; roles in bioenergetics and cell survival. J Bioenerg Biomembr 46, 1–15 (2014). https://doi.org/10.1007/s10863-013-9527-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-013-9527-7