Abstract
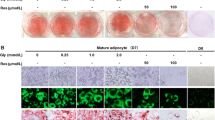
NYGGF4 (also called PID1) was demonstrated that it may be related to the development of obesity-related IR. We aimed in the present study to further elucidate the effects of NYGGF4 on IR and the underlying mechanisms through using α-Lipoic acid (LA) treatment, which could facilitate glucose transport and utilization in fully differentiated adipocytes. Our data showed that the LA pretreatment strikingly enhanced insulin-stimulated glucose uptake through increasing GLUT4 translocation to the PM in NYGGF4 overexpression adipocytes. The reactive oxygen species (ROS) levels in NYGGF4 overexpression adipocytes were strikingly enhanced, which could be decreased by the LA pretreatment. NYGGF4 overexpression resulted in significant inhibition of tyrosine phosphorylation of IRS-1 and serine phosphorylation of Akt, whereas incubation with LA strongly activated IRS-1 and Akt phosphorylation in NYGGF4 overexpression adipocytes. These results suggest that LA protects 3T3-L1 adipocytes from NYGGF4-induced IR partially through increasing phosphorylation of IRS-1 and Akt and provide evidence that NYGGF4 may be a potential target for the treatment of obesity and obesity-related IR.
Similar content being viewed by others
References
Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT et al (2009) Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119(3):573–581
Andreozzi F, Laratta E, Sciacqua A, Perticone F, Sesti G (2004) Angiotensin II impairs the insulin signaling pathway promoting production of nitric oxide by inducing phosphorylation of insulin receptor substrate-1 on Ser312 and Ser616 in human umbilical vein endothelial cells. Circ Res 94(9):1211–1218
Andreyev AY, Kushnareva YE, Starkov AA (2005) Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 70(2):200–214
Barja G (1999) Mitochondrial oxygen radical generation and leak: sites of production in states 4 and 3, organ specificity, and relation to aging and longevity. J Bioenerg Biomembr 31(4):347–366
Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B et al (2008) Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 118(2):789–800
Bryant NJ, Govers R, James DE (2002) Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol 3(4):267–277
Caratù G, Allegra D, Bimonte M, Schiattarella GG, D’Ambrosio C, Scaloni A et al (2007) Identification of the ligands of protein interaction domains through a functional approach. Mol Cell Proteomics 6(2):333–345
Ceddia RB, Somwar R, Maida A, Fanq X, Bikopouls G, Sweeney G (2005) Globular adiponectin increases GLUT4 translocation and glucose uptake but reduces glycogen synthesis in rat skeletal muscle cells. Diabetoloqia 48(1):132–139
Cheng Z, White MF (2010) Foxo1 in hepatic lipid metabolism. Cell Cycle 9(2):219–220
Cheng Z, Guo S, Copps K, Dong X, Kollipara R, Rodgers JT et al (2009) Foxo1 integrates insulin signaling with mitochondrial function in the liver. Nat Med 15(11):1307–1311
Czech MP, Corvera S (1999) Signaling mechanisms that regulate glucose transport. J Biol Chem 274(4):1865–1868
de Jongh RT, Serné EH, Ijzerman RG, de Vries G, Stehouwer CD (2004) Free fatty acid levels modulate microvascular function: relevance for obesity-associated insulin resistance, hypertension, and microangiopathy. Diabetes 53(11):2873–2882
Ducluzeau PH, Fletcher LM, Vidal H, Laville M, Tavaré JM (2002) Molecular mechanisms of insulin-stimulated glucose uptake in adipocytes. Diabetes Metab 28(2):85–92
Eason RC, Archer HE, Akhtar S, Bailey CJ (2002) Lipoic acid increases glucose uptake by skeletal muscles of obese-diabetic ob/ob mice. Diabetes Obes Metab 4(1):29–35
Estrada DE, Ewart HS, Tsakiridis T, Volchuk A, Ramlal T, Tritschler H et al (1996a) Stimulation of glucose uptake by the natural coenzyme alpha-lipoic acid/thioctic acid: participation of elements of the insulin signaling pathway. Diabetes 45(12):1798–1804
Estrada DE, Ewart HS, Tsakiridis T, Volchuk A, Ramlal T, Tritschler H et al (1996b) Diabetes 45(12):1798–1804
Ferrannini E, Balkau B, Coppack SW, Dekker JM, Mari A, Nolan J et al (2007) Insulin resistance, insulin response, and obesity as indicators of metabolic risk. J Clin Endocrinol Metab 92(8):2885–2892
Guilherme A, Virbasius JV, Puri V, Czech MP (2008) Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 9(5):367–377
Herz J, Strickland DK (2001) LRP: a multifunctional scavenger and signaling receptor. J Clin Invest 108(6):779–784
Houstis N, Rosen ED, Lander ES (2006) Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440(7086):944–948
Jackson C (2006) Diabetes: kicking off the insulin cascade. Nature 444(7121):833–834
Jacob S, Henriksen EJ, Schiemann AL, Simon I, Clancy DE, Tritschler HJ et al (1995) Enhancement of glucose disposal in patients with type 2 diabetes by alpha-lipoic acid. Arzneimittelforschung 45(8):872–874
Jacob S, Henriksen EJ, Tritschler HJ, Augustin HJ, Dietze GJ (1996) Improvement of insulin-stimulated glucose-disposal in type 2 diabetes after repeated parenteral administration of thioctic acid. Exp Clin Endocrinol Diabetes 104(3):284–288
Jacob S, Ruus P, Hermann R, Tritschler HJ, Maerker E, Renn W et al (1999) Oral administration of RAC-alpha-lipoic acid modulates insulin sensitivity in patients with type-2 diabetes mellitus: a placebo-controlled pilot trial. Free Rad Biol Med 27(3–4):309–314
Kahn CR (1978) Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metabolism 27(12 Suppl 2):1893–1902
Kanzaki M (2006) Insulin receptor signals regulating GLUT4 translocation and actin dynamics. Endocr J 53(3):267–293
Kim JA, Wei Y, Sowers JR (2008) Role of mitochondrial dysfunction in insulin resistance. Circ Res 102(4):401–414
Konrad D, Somwar R, Sweeney G, Yaworsky K, Hayashi M, Ramlal T et al (2001) The antihyperglycemic drug alpha-lipoic acid stimulates glucose uptake via both GLUT4 translocation and GLUT4 activation: potential role of p38 mitogen-activated protein kinase in GLUT4 activation. Diabetes 50(6):1464–1471
Kremen J, Dolinkova M, Krajickova J, Blaha J, Anderlova K, Lacinova Z et al (2006) Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: possible role in postoperative insulin resistance. J Clin Endocrinol Metab 91(11):4620–4627
Liu S, Okada T, Assmann A, Soto J, Liew CW, Bugger H et al (2009) Insulin signaling regulates mitochondrial function in pancreatic beta-cells. PLoS One 4(11):e7983
Moini H, Tirosh O, Park YC, Cho KJ, Packer L (2002a) R-alpha-lipoic acid action on cell redox status, the insulin receptor, and glucose uptake in 3T3-L1 adipocytes. Arch Biochem Biophys 397(2):384–391
Moini H, Tirosh O, Park YC, Cho KJ, Packer L (2002b) R-a-Lipoic acid action on cell redox status, the insulin receptor, and glucose uptake in 3T3-L1 adipocytes. Arch Biochem Biophys 397(2):384–391
O'Neill BT, Kim J, Wende AR, Theobald HA, Tuinei J, Buchanan J et al (2007) A conserved role for phosphatidylinositol 3-kinase but not Akt signaling in mitochondrial adaptations that accompany physiological cardiac hypertrophy. Cell Metab 6(4):294–306
Qatanani M, Lazar MA (2007) Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev 21(12):1443–1455
Ramath S, Tritschler HJ, Eckel J (1999) Horm Metab Res 31(12):632–635
Reaven GM (1988) Role of insulin resistance in human disease. Diabetes 37(12):1595–1607
Schrauwen P, Hesselink MK (2004) Oxidative capacity, lipotoxicity, and mitochondrial damage in type 2 diabetes. Diabetes 53(6):1412–1417
Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T (1995) Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270(5234):296–299
Tapodi A, Debreceni B, Hanto K, Bognar Z, Wittmann I, Gallyas F Jr et al (2005) Pivotal role of Akt activation in mitochondrial protection and cell survival by poly (ADPribose) polymerase-1 inhibition in oxidative stress. J Biol Chem 280(42):35767–35775
Tilg H, Moschen AR (2006) Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6(10):772–783
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 522(Pt 2):335–344
Wang B, Zhang M, Ni YH, Liu F, Fan HQ, Fei L et al (2006) Identification and characterization of NYGGF4, a novel gene containing a phosphotyrosine-binding (PTB) domain that stimulates 3T3-L1 preadipocytes proliferation. Gene 379:132–140
Wu WL, Gan WH, Tong ML, Li XL, Dai JZ, Zhang CM et al (2011) Over-expression of NYGGF4 (PID1) inhibits glucose transport in skeletal myotubes by blocking the IRS1/PI3K/AKT insulin pathway. Mol Genet Metab 102(3):374–377
Yaworsky K, Somwar R, Ramlal T, Tritschler HJ, Klip A (2000a) Engagement of the insulin-sensitive pathway in the stimulation of glucose transport by alpha-lipoic acid in 3T3-L1 adipocytes. Diabetologia 43(3):294–303
Yaworsky K, Somwar R, Ramlal T, Tritschler HJ, Klip A, Yaworsky K (2000b) Engagement of the insulin-sensitive pathway in the stimulation of glucose transport by alpha-lipoic acid in 3T3-L1 adipocytes. Diabetologia 43(3):294–303
Yonezawa K, Ueda H, Hara K, Nishida K, Ando A, Chavanieu A et al (1992) Insulin-dependent formation of a complex containing an 85-kDa subunit of phosphatidylinositol 3-kinase and tyrosine-phosphorylated insulin receptor substrate 1. J Biol Chem 267(36):25958–25965
Zhang CM, Chen XH, Wang B, Liu F, Chi X, Tong ML et al (2009) Over-expression of NYGGF4 inhibits glucose transport in 3T3-L1 adipocytes via attenuated phosphorylation of IRS-1 and Akt. Acta Pharmacol Sin 30(1):120–124
Zhang CM, Zeng XQ, Zhang R, Ji CB, Tong ML, Chi X et al (2010) Effects of NYGGF4 knockdown on insulin sensitivity and mitochondrial function in 3T3-L1 adipocytes. J Bioenerg Biomembr 42(5):433–439
Zhao Y, Zhang C, Chen X, Gao C, Ji C, Chen F et al (2010) Overexpression of NYGGF4 (PID1) induces mitochondrial impairment in 3T3-L1 adipocytes. Mol Cell Biochem 340(1–2):41–48
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yu-mei Wang and Xiao-fei Lin contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, Ym., Lin, Xf., Shi, Cm. et al. α-Lipoic acid protects 3T3-L1 adipocytes from NYGGF4 (PID1) overexpression-induced insulin resistance through increasing phosphorylation of IRS-1 and Akt. J Bioenerg Biomembr 44, 357–363 (2012). https://doi.org/10.1007/s10863-012-9440-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-012-9440-5