Abstract
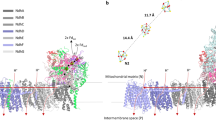
The photosynthetic oxygen evolving complex (PSII-OEC) and the mitochondrial cytochrome c oxidase (CcO) not only catalyze anti-parallel reactions (the OEC oxidizes water to dioxygen, whereas CcO reduces dioxygen to water), they also share a number of uncanny molecular and mechanistic similarities. Both feature a redox-active polymetallic cluster that includes a key tyrosine, and both utilize a two-phase mechanism. In one phase the polymetallic cluster undergoes four sequential one-electron transfers: In the PSII-OEC, four successive photooxidations of the photosystem II reaction center P680 (to P680+) allows acceptance of 4 × 1e- from the Mn4Ca cluster; in CcO, four reduced cytochrome c Fe2+ cations donate 4 × 1e- to the bimetallic center. In the second phase for each enzyme, the polymetallic cluster undergoes a single four-electron transfer with the O2/2 H2O redox couple. Intriguing mechanistic similarities between these two complex redox enzymes first delineated over a decade ago by Hoganson/Proshlyakov/Babcock et al. are updated and expanded in this article.
Similar content being viewed by others
References
Affourtit C, Albury MS, Crichton PG, Moore AL (2002) Exploring the mole-cular nature of alternative oxidase regulation and catalysis. FEBS Lett 510:121–126
Ahlbrink R, Haumann M, Cherepanov D, Bögershausen O, Mulkidjanian A, Junge W (1998) Function of tyrosine Z in water oxidation by photosystem II: electrostatical promotor instead of hydrogen abstractor. Biochem 37(4):1131–1142
Babcock GT (1999) How oxygen is activated and reduced in respiration. Proc Natl Acad Sci U S A 96(23):13114–13117
Babcock GT, Wikstrom M (1992) O2 activation and the conservation of energy in cell respiration. Nature 356:301–309
Babcock GT, Wikström M (1992) Oxygen activation and the conservation of energy in cell respiration. Nature 356:301–309
Babcock GT, Barry BA, Debus RJ, Hoganson CW, Atamian M, McIntosh L, Sithole I, Yocum CF (1989) Water oxidation in photosystem II: from radical chemistry to multielectron chemistry. Biochem 28:9557–9565
Belevich I, Verkhovsky MI, Wikstrom M (2006) Proton-coupled electron transfer drives the proton pump of cytochrome c oxidase. Nature 440:829–832
Berg JM, Tymoczko JL, Stryer L (2007) Biochemistry, 6th edn., W. H. Freeman, NY; pp. 514–517 and 549–551
Clausen J, Junge W (2004) Detection of an intermediate of photosynthetic water oxidation. Nature 430:480–483
Cook CD, Depatie CB (1959) Oxidation of hindered phenols. VIII. Kinetics of the oxidation of 2,4,6-tri-t-butylphenol by benzoyl peroxide. J Org Chem 24:1144–1146
Dau H, Haumann M (2006) Photosynthetic oxygen production: response. Science 312:1471–1472
Dau H, Haumann M (2008) The manganese complex of photosytem II in its reaction cycle—basic framework and possible realization at the atomic level. Coord Chem Rev 252:273–295
De AK, Dutta BK, Bhattacharjee S (2006) Reaction kinetics for the degradation of phenol and chlorinated phenols using Fenton’s reagent. Env Program 25:64–71
Haumann M, Liebisch P, Muller C, Barra M, Grabolle M, Dau H (2005) Photosynthetic O2 formation tracked by time-resolved x-ray experiments. Science 310:1019–1021
Haumann M, Grundmeier A, Zaharieva I, Dau H (2008) Photosynthetic water oxidation at elevated dioxygen partial pressure monitored by time-resolved X-ray absorption measurements. Proc Natl Acad Sci U S A 105(45):17384–17389
Hays A-MA, Vassiliev IR, Golbeck JH, Debus RJ (1998) Role of D1-His190 in proton-coupled electron transfer reactions in photosystem II: a chemical complementation study. Biochem 37:11352–11365
Hoganson CW, Babcock GT (1997) A metalloradical mechanism for the generation of oxygen from water in photosynthesis. Science 277:1953–1956
Hoganson CW, Pressler MA, Proshlyakov DA, Babcock GT (1998) From water to oxygen and back again: mechanistic similarities in the enzymatic redox conversions between water and dioxygen. Biochim Biophys Acta 1365:170–174
Howard DL, Tinoco AD, Brudvig GW, Vrettos JS, Allen BC (2005) Catalytic oxygen evolution by a bioinorganic model of the photosystem II oxygen-evolving complex. J Chem Educ 82:791–794
Joliot P, Kok B (1975) Oxygen evolution in photosynthesis. In: Govindjee (ed) Bio-energetics of photosynthesis. Academic, London, pp 387–411
Kok B, Forbush B, McGloin M (1970) Cooperation of charges in photosynthetic oxygen evolution. I: a linear four-step mechanism. Photochem Photobiol 11:457–475
Mamedov F, Sayre RT, Styring S (1998) Involvement of histidine 190 on the D1 protein in electron/proton transfer reactions on the donor side of photosystem II. Biochem 37:14245–14256
Marechal M, Kido Y, Kita K, Moore AL, Rich PR (2009) Three redox states of Trypanosoma brucei alternative oxidase identified by infrared spectroscopy and electrochemistry. J Biol Chem 284:31827–31833
McEvoy JP, Brudvig GW (2004) Structure-based mechanism of photosynthetic water oxidation. Phys Chem Chem Phys 6:4754–4763
Moore AL, Carre JE, Affourtit C, Albury MS, Crichton PG, Kita K, Heathcote P (2008) Compelling EPR evidence that the alternative oxidase is a diiron carboxylate protein. Biochim Biophys Acta 1777:327–330
Pignatello JJ (1992) Dark and photoassisted iron(3+)-catalyzed degradation of chlorophenoxy herbicides by hydrogen peroxide. Environ Sci Tech 26:944–951
Proshlyakov DA, Pressler MA, Babcock GT (1998) Dioxygen activation and bond cleavage by mixed-valence cytochrome c oxidase. Proc Natl Acad Sci U S A 95:8020–8025
Rappaport F, Lavergne J (1997) Charge recombination and proton transfer in manganese-depleted photosystem II. Biochemistry 36:15294–15302
Rappaport F, Guergova-Kuras M, Nixon PJ, Diner BA, Lavergne J (2002) Kinetics and pathways of charge recombination in photosystem II. Biochemistry 41:8518–8527
Silverstein TP et al (1993) Transmembrane measurements across bioenergetic membranes. Biochim Biophys Acta 1183:1–3
Sproviero EM, Gascon JA, McEvoy JP, Brudvig GW, Batista VS (2008) Quantum mechanics/molecular mechanics study of the catalytic cycle of water splitting in photosystem II. J Am Chem Soc 130:3428–3442
Svensson-Elk M, Abramson J, Carsson G, Tornoth S, Brzezinski P, Iwata S (2002) The x-ray crystal structures of wild-type and EQ(I-286) mutant cytochrome c oxidases from Rhodobacter sphaeroides. J Mol Biol 321:329–339
Tommos C, Babcock G (1998) Oxygen production in nature: a light-driven metalloradical enzyme process. Acc Chem Res 31:18–25
Umena Y, Kawakami K, Shen J-R, Kamiya N (2011) Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473:55–60
Voet D, Voet JG, Pratt CW (2008) Fundamentals of biochemistry, 3rd edn., Wiley, pp. 615–618 and 654–656
Wagner AM, Moore AL (1997) Structure and function of the plant alternative oxidase: its putative role in the oxygen defence mechanism. Biosci Rep 17:319–333
Wikstrom MKF (1977) Proton pump coupled to cytochrome c oxidase in mitochondria. Nature 266:271–273
Wikstrom M, Verkhovsky MI (2006) Towards a mechanism of proton pumping by the haem-copper oxidases. Biochim Biophys Acta 1757:1047–1051
Yano J et al (2006) Where water is oxidized to dioxygen: structure of the photosynthetic Mn4Ca cluster. Science 314:821–825
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silverstein, T.P. Photosynthetic water oxidation vs. mitochondrial oxygen reduction: distinct mechanistic parallels. J Bioenerg Biomembr 43, 437–446 (2011). https://doi.org/10.1007/s10863-011-9370-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-011-9370-7