Abstract
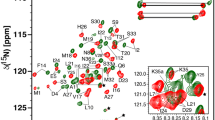
N-linked glycosylation is an essential and highly conserved co- and post-translational protein modification in all domains of life. In humans, genetic defects in N-linked glycosylation pathways result in metabolic diseases collectively called Congenital Disorders of Glycosylation. In this modification reaction, a mannose rich oligosaccharide is transferred from a lipid-linked donor substrate to a specific asparagine side-chain within the -N-X-T/S- sequence (where X ≠ Proline) of the nascent protein. Oligosaccharyltransferase (OST), a multi-subunit membrane embedded enzyme catalyzes this glycosylation reaction in eukaryotes. In yeast, Ost4 is the smallest of nine subunits and bridges the interaction of the catalytic subunit, Stt3, with Ost3 (or its homolog, Ost6). Mutations of any C-terminal hydrophobic residues in Ost4 to a charged residue destabilizes the enzyme and negatively impacts its function. Specifically, the V23D mutation results in a temperature-sensitive phenotype in yeast. Here, we report the reconstitution of both purified recombinant Ost4 and Ost4V23D each in a POPC/POPE lipid bilayer and their resonance assignments using heteronuclear 2D and 3D solid-state NMR with magic-angle spinning. The chemical shifts of Ost4 changed significantly upon the V23D mutation, suggesting a dramatic change in its chemical environment.




Similar content being viewed by others
References
Bai L, Wang T, Zhao G, Kovach A, Li H (2018) The atomic structure of a eukaryotic oligosaccharyltransferase complex. Nature 555(7696):328–333. https://doi.org/10.1038/nature25755
Brown MF (1994) Modulation of rhodopsin function by properties of the membrane bilayer. Chem Phys Lipids 73(1–2):159–180. https://doi.org/10.1016/0009-3084(94)90180-5
Carman GM, Han GS (2009) Regulation of phospholipid synthesis in yeast. J Lipid Res 50(Suppl):S69–S73. https://doi.org/10.1194/jlr.R800043-JLR200
Chaudhary B, Mazumder S, Mohanty S (2017) Production and biophysical characterization of a mini-membrane protein, Ost4V23D: a functionally important mutant of yeast oligosaccharyltransferase subunit Ost4p. Protein Expr Purif 139:43–48. https://doi.org/10.1016/j.pep.2017.07.009
Chaudhary BP, Zoetewey D, Mohanty S (2020) 1H, 13C, 15N resonance assignments and secondary structure of yeast oligosaccharyltransferase subunit Ost4 and its functionally important mutant Ost4V23D. Biomol NMR Assign 14(2):205–209. https://doi.org/10.1007/s12104-020-09946-7
Chaudhary BP, Zoetewey DL, McCullagh MJ, Mohanty S (2021) NMR and MD simulations reveal the impact of the V23D mutation on the function of yeast oligosaccharyltransferase subunit Ost4. Glycobiology 31(7):838–850. https://doi.org/10.1093/glycob/cwab002
Cherepanova N, Shrimal S, Gilmore R (2016) N-linked glycosylation and homeostasis of the endoplasmic reticulum. Curr Opin Cell Biol 41:57–65. https://doi.org/10.1016/j.ceb.2016.03.021
Chi JH, Roos J, Dean N (1996) The OST4 gene of Saccharomyces cerevisiae encodes an unusually small protein required for normal levels of oligosaccharyltransferase activity. J Biol Chem 271(6):3132–3140. https://doi.org/10.1074/jbc.271.6.3132
Das N, Murray DT, Cross TA (2013) Lipid bilayer preparations of membrane proteins for oriented and magic-angle spinning solid-state NMR samples. Nat Protoc 8(11):2256–2270. https://doi.org/10.1038/nprot.2013.129
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6(3):277–293. https://doi.org/10.1007/BF00197809
Dempski RE, Imperiali B (2002) Oligosaccharyl transferase: gatekeeper to the secretory pathway. Curr Opin Chem Biol 6(6):844–850. https://doi.org/10.1016/s1367-5931(02)00390-3
Epand RM, Lester DS (1990) The role of membrane biophysical properties in the regulation of protein kinase C activity. Trends Pharmacol Sci 11(8):317–320. https://doi.org/10.1016/0165-6147(90)90234-y
Escribá PV, Ozaita A, Ribas C, Miralles A, Fodor E, Farkas T, García-Sevilla JA (1997) Role of lipid polymorphism in G protein-membrane interactions: nonlamellar-prone phospholipids and peripheral protein binding to membranes. Proc Natl Acad Sci U S A 94(21):11375–11380. https://doi.org/10.1073/pnas.94.21.11375
Franks WT, Kloepper KD, Wylie BJ, Rienstra CM (2007) Four-dimensional heteronuclear correlation experiments for chemical shift assignment of solid proteins. J Biomol NMR 39(2):107–131. https://doi.org/10.1007/s10858-007-9179-1
Freeze HH (2013) Understanding human glycosylation disorders: biochemistry leads the charge. J Biol Chem 288(10):6936–6945. https://doi.org/10.1074/jbc.R112.429274
Gahmberg CG, Tolvanen M (1996) Why mammalian cell surface proteins are glycoproteins. Trends Biochem Sci 21(8):308–311
Gayen S, Kang C (2011) Solution structure of a human minimembrane protein Ost4, a subunit of the oligosaccharyltransferase complex. Biochem Biophys Res Commun 409(3):572–576. https://doi.org/10.1016/j.bbrc.2011.05.050
Gopinath T, Weber D, Wang S, Larsen E, Veglia G (2021) Solid-state NMR of membrane proteins in lipid bilayers: to spin or not to spin? Acc Chem Res 54(6):1430–1439. https://doi.org/10.1021/acs.accounts.0c00670
Helenius A, Aebi M (2004) Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem 73:1019–1049. https://doi.org/10.1146/annurev.biochem.73.011303.073752
Henderson CM, Block DE (2014) Examining the role of membrane lipid composition in determining the ethanol tolerance of Saccharomyces cerevisiae. Appl Environ Microbiol 2966–2972. https://doi.org/10.1128/AEM.04151-13
Hennet T, Cabalzar J (2015) Congenital disorders of glycosylation: a concise chart of glycocalyx dysfunction. Trends Biochem Sci 40(7):377–384. https://doi.org/10.1016/j.tibs.2015.03.002
Huang C, Bhaskaran R, Mohanty S (2012) Eukaryotic N-glycosylation occurs via the membrane-anchored C-terminal domain of the Stt3p subunit of oligosaccharyltransferase. J Biol Chem 287(39):32450–32458. https://doi.org/10.1074/jbc.M112.342253
Karaoglu D, Kelleher DJ, Gilmore R (1997) The highly conserved Stt3 protein is a subunit of the yeast oligosaccharyltransferase and forms a subcomplex with Ost3p and Ost4p. J Biol Chem 272(51):32513–32520. https://doi.org/10.1074/jbc.272.51.32513
Kelleher DJ, Gilmore R (2006) An evolving view of the eukaryotic oligosaccharyltransferase. Glycobiology 16(4):47R–62. https://doi.org/10.1093/glycob/cwj066. R
Kim H, Park H, Montalvo L, Lennarz WJ (2000) Studies on the role of the hydrophobic domain of Ost4p in interactions with other subunits of yeast oligosaccharyl transferase. Proc Natl Acad Sci U S A 97(4):1516–1520. https://doi.org/10.1073/pnas.040556797
Kim H, Yan Q, Von Heijne G, Caputo GA, Lennarz WJ (2003) Determination of the membrane topology of Ost4p and its subunit interactions in the oligosaccharyltransferase complex in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 100(13):7460–7464. https://doi.org/10.1073/pnas.1332735100
Knauer R, Lehle L (1999) The oligosaccharyltransferase complex from yeast. Biochim Biophys Acta 1426(2):259–273. https://doi.org/10.1016/s0304-4165(98)00128-7
Kornfeld R, Kornfeld S (1985) Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem 54:631–664. https://doi.org/10.1146/annurev.bi.54.070185.003215
Kumar A, Ward P, Katre UV, Mohanty S (2012) A novel and simple method of production and biophysical characterization of a mini-membrane protein, Ost4p: a subunit of yeast oligosaccharyl transferase. Biopolymers 97(7):499–507. https://doi.org/10.1002/bip.22028
Larkin A, Imperiali B (2011) The expanding horizons of asparagine-linked glycosylation. Biochemistry 50(21):4411–4426. https://doi.org/10.1021/bi200346n
Marsh JA, Singh VK, Jia Z, Forman-Kay JD (2006) Sensitivity of secondary structure propensities to sequence differences between alpha- and gamma-synuclein: implications for fibrillation. Protein Sci 15(12):2795–2804. https://doi.org/10.1110/ps.062465306
Mohanty S, Chaudhary BP, Zoetewey D (2020) Structural insight into the mechanism of. Biomolecules 10(4). https://doi.org/10.3390/biom10040624
Monje-Galvan V, Klauda JB (2015) Modeling yeast organelle membranes and how lipid diversity influences Bilayer Properties. Biochemistry 54(45):6852–6861. https://doi.org/10.1021/acs.biochem.5b00718
Mueller S, Wahlander A, Selevsek N, Otto C, Ngwa EM, Poljak K, Gauss R (2015) Protein degradation corrects for imbalanced subunit stoichiometry in OST complex assembly. Mol Biol Cell 26(14):2596–2608. https://doi.org/10.1091/mbc.E15-03-0168
Pauli J, Baldus M, van Rossum B, de Groot H, Oschkinat H (2001) Backbone and side-chain 13 C and 15 N signal assignments of the alpha-spectrin SH3 domain by magic angle spinning solid-state NMR at 17.6 Tesla. ChemBioChem 2(4):272–281. https://doi.org/10.1002/1439-7633(20010401)2:4<272::AID-CBIC272>3.0.CO;2-2
Ramírez AS, Kowal J, Locher KP (2019) Cryo-electron microscopy structures of human oligosaccharyltransferase complexes OST-A and OST-B. Science 366(6471):1372–1375. https://doi.org/10.1126/science.aaz3505
Schulz BL, Stirnimann CU, Grimshaw JP, Brozzo MS, Fritsch F, Mohorko E, Aebi M (2009) Oxidoreductase activity of oligosaccharyltransferase subunits Ost3p and Ost6p defines site-specific glycosylation efficiency. Proc Natl Acad Sci U S A 106(27):11061–11066. https://doi.org/10.1073/pnas.0812515106
Stevens TJ, Fogh RH, Boucher W, Higman VA, Eisenmenger F, Bardiaux B, Laue ED (2011) A software framework for analysing solid-state MAS NMR data. J Biomol NMR 51(4):437–447. https://doi.org/10.1007/s10858-011-9569-2
Takegoshi K, Nakamura S, Terao T (2001) 13 C–1H dipolar-assisted rotational resonance in magic-angle spinning NMR. Chem Phys Lett 344(5):631–637. https://doi.org/10.1016/S0009-2614(01)00791-6
van ‘t Klooster JS, Cheng TY, Sikkema HR, Jeucken A, Moody DB, Poolman B (2020) Membrane lipid requirements of the lysine transporter Lyp1 from Saccharomyces cerevisiae. J Mol Biol 432(14):4023–4031. https://doi.org/10.1016/j.jmb.2020.04.029
Wacker M, Linton D, Hitchen PG, Nita-Lazar M, Haslam SM, North SJ, Aebi M (2002) N-linked glycosylation in Campylobacter jejuni and its functional transfer into E. Coli. Science 298(5599):1790–1793. https://doi.org/10.1126/science.298.5599.1790
Welply JK, Shenbagamurthi P, Lennarz WJ, Naider F (1983) Substrate recognition by oligosaccharyltransferase. Studies on glycosylation of modified Asn-X-Thr/Ser tripeptides. J Biol Chem 258(19):11856–11863
Wild R, Kowal J, Eyring J, Ngwa EM, Aebi M, Locher KP (2018) Structure of the yeast oligosaccharyltransferase complex gives insight into eukaryotic N-glycosylation. Science 359(6375):545–550. https://doi.org/10.1126/science.aar5140
Zhao H, Michelot A, Koskela EV, Tkach V, Stamou D, Drubin DG, Lappalainen P (2013) Membrane-sculpting BAR domains generate stable lipid microdomains. Cell Rep 4(6):1213–1223. https://doi.org/10.1016/j.celrep.2013.08.024
Zubkov S, Lennarz WJ, Mohanty S (2004) Structural basis for the function of a minimembrane protein subunit of yeast oligosaccharyltransferase. Proc Natl Acad Sci U S A 101(11):3821–3826. https://doi.org/10.1073/pnas.0400512101
Acknowledgements
This work was financially supported by National Science Foundation Award CHE-1807722 and DBI-1726397 to SM. We thank Salik R Dahal and Omar Al-Danoon for their help during the protein expression and reconstitution. We thank Dr Thomas Webb from Auburn University, Auburn, AL for critical reading to improve the manuscript quality.
Author information
Authors and Affiliations
Contributions
SM conceived, designed the strategies and the techniques used for the research, and supervised the research. BC expressed, purified both the recombinant proteins. BC, HM, and DZhou reconstituted the proteins into the lipid bilayer. JS performed all the ssNMR experiments. BC processed, analyzed the NMR data, and completed resonance assignments. SM and DZoetewey did some of the analysis. SM, DZoetewey, and BC wrote the paper, and BC prepared all the figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chaudhary, B.P., Struppe, J., Moktan, H. et al. Reconstitution and resonance assignments of yeast OST subunit Ost4 and its critical mutant Ost4V23D in liposomes by solid-state NMR. J Biomol NMR (2024). https://doi.org/10.1007/s10858-024-00437-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10858-024-00437-8