Abstract
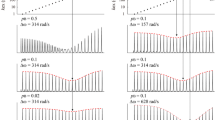
NOEs between the β-protons of cysteine residues across disulfide bonds in proteins provide direct information on the connectivities and conformations of these important cross-links, which are otherwise difficult to investigate. With conventional [U-13C, 15N]-proteins, however, fast spin diffusion processes mediated by strong dipolar interactions between geminal β-protons prohibit the quantitative measurements and thus the analyses of long-range NOEs across disulfide bonds. We describe a robust approach for alleviating such difficulties, by using proteins selectively labeled with an equimolar mixture of (2R, 3S)-[β-13C; α,β-2H2] Cys and (2R, 3R)-[β-13C; α,β-2H2] Cys, but otherwise fully deuterated. Since either one of the prochiral methylene protons, namely β2 (proS) or β3 (proR), is always replaced with a deuteron and no other protons remain in proteins prepared by this labeling scheme, all four of the expected NOEs for the β-protons across disulfide bonds could be measured without any spin diffusion interference, even with long mixing times. Therefore, the NOEs for the β2 and β3 pairs across each of the disulfide bonds could be observed at high sensitivity, even though they are 25% of the theoretical maximum for each pair. With the NOE information, the disulfide bond connectivities can be unambiguously established for proteins with multiple disulfide bonds. In addition, the conformations around disulfide bonds, namely χ2 and χ3, can be determined based on the precise proton distances of the four β-proton pairs, by quantitative measurements of the NOEs across the disulfide bonds. The feasibility of this method is demonstrated for bovine pancreatic trypsin inhibitor, which has three disulfide bonds.








Similar content being viewed by others
References
Altman JD, Henner D, Nilsson B, Anderson S, Kuntz ID (1991) Intracellular expression of BPTI fusion proteins and single column cleavage/affinity purification by chymotrypsin. Protein Eng 4:593–600
Arnold LD, Kalantar TH, Vederas JC (1985) Conversion of serine to stereochemically pure β-substituted α-amino acids via β-lactones. J Am Chem Soc 107:7105–7109
Arnold LD, May RG, Vederas JC (1988) Synthesis of optically pure α-amino acids via salts of α-amino-β-propiolactone. J Am Chem Soc 110:2237–2241
Berndt KD, Güntert P, Orbons LP, Wüthrich K (1992) Determination of a high-quality nuclear magnetic resonance solution structure of the bovine pancreatic trypsin inhibitor and comparison with three crystal structures. J Mol Biol 277:757–775
Billeter M, Braun W, Wüthrich K (1982) Sequential resonance assignments in protein 1H nuclear magnetic resonance spectra. Computation of sterically allowed proton–proton distances and statistical analysis of proton–proton distances in single crystal protein conformations. J Mol Biol 155:321–346
Boyd J, Hommel U, Campbell ID (1990) Influence of cross-correlation between dipolar and anisotropic chemical shift relaxation mechanisms upon longitudinal relaxation rates of 15N in macromolecules. Chem Phys Lett 175:477–482
Brüeschweiler R, Roux B, Blackledge M, Griesinger C, Karplus M, Ernst RR (1992) Influence of rapid intramolecular motion on NMR cross-relaxation rates. A molecular dynamics study of Antamanide in solution. J Am Chem Soc 114:2289–2302
Cavanagh J, Fairbrother WJ, Palmer AG, Skelton NJ, Rance M (2007) Protein NMR spectroscopy: principles and practice. Academic Press, San Diego
Creighton TE (1984) Disulfide bond formation in proteins. Methods Enzymol 107:305–329
Goerke AR, Swartz JR (2008) Development of cell-free protein synthesis platforms for disulfide bonded proteins. Biotechnol Bioeng 99:351–367
Grey MJ, Wang C, Parmer AG (2003) Disulfide bond isomerization in basic pancreatic trypsin inhibitor: multisite chemical exchange quantified by CPMG relaxation dispersion and chemical shift modeling. J Am Chem Soc 125:14324–14335
Ikeya T, Takeda M, Yoshida H, Terauchi T, Jee JG, Kainosho M, Güntert P (2009) Automated NMR structure determination of stereo-array isotope labeled ubiquitin from minimal sets of spectra using the SAIL-FLYA system. J Biomol NMR 44:261–272
Kainosho M, Güntert P (2009) SAIL—stereo-array isotope labeling. Q Rev Biophys 42:247–300
Kainosho M, Torizawa T, Iwashita Y, Terauchi T, Ono AM, Güntert P (2006) Optimal isotope labelling for NMR protein structure determinations. Nature 440:52–57
Kari C, Nagy Z, Kovács P, Hernádi F (1971) Mechanism of the growth inhibitory effect of cysteine on Escherichia coli. J Gen Microbiol 68:349–356
Kay LE, Torchia DA, Bax A (1989) Backbone dynamics of proteins as studied by 15N inverse detected heteronuclear NMR spectroscopy: application to staphylococcal nuclease. Biochemistry 28:8972–8979
Kojima C, Ono A, Kainosho M, James TL (1998) DNA duplex dynamics: NMR relaxation studies of a decamer with uniformly 13C-labeled purine nucleotides. J Magn Reson 135:310–333
Kumar A, Wagner G, Ernst RR, Wüthrich K (1981) Buildup rates of the nuclear Overhauser effect measured by two-dimensional proton magnetic resonance spectroscopy: implications for studies of protein conformation. J Am Chem Soc 103:3654–3658
Lane AN (1988) The influence of spin diffusion and internal motions on NOE intensities in proteins. J Magn Reson 78:425–439
LeMaster DM (1996) Structural determinants of the catalytic reactivity of the buried cysteine of Escherichia coli thioredoxin. Biochemistry 35:14876–14881
Lipari G, Szabo A (1982a) Model-free approach to the interpretation of nuclear magnetic resonance relaxation in macromolecules. 1. Theory and range of validity. J Am Chem Soc 104:4546–4559
Lipari G, Szabo A (1982b) Model-free approach to the interpretation of nuclear magnetic resonance relaxation in macromolecules. 2. Analysis of experimental results. J Am Chem Soc 104:4559–4570
Macura S, Fejzo J, Westler WM, Markley JL (1994) Influence of slow internal motion in proteins on cross-relaxation rates determined by two-dimensional exchange spectroscopy. Bull Magn Reson 16:73–93
Markley JL, Bax A, Arata Y, Hilbers CW, Kaptein R, Sykes BD, Wright PE, Wüthrich K (1998) Recommendations for the presentation of NMR structures of proteins and nucleic acids. J Mol Biol 280:933–952
Matsumura M, Matthews BW (1991) Stabilization of functional proteins by introduction of multiple disulfide bonds. Methods Enzymol 202:336–356
Miyanoiri Y, Takeda M, Jee J, Ono AM, Okuma K, Terauchi T, Kainosho M (2011) Alternative SAIL-Trp for robust aromatic signal assignment and determination of the χ(2) conformation by intra-residue NOEs. J Biomol NMR. doi:10.1007/s10858-011-9568-3 (in press)
Mobli M, King GF (2010) NMR methods for determining disulfide-bond connectivities. Toxicon 56:849–854
Mobli M, de Araújo AD, Lambert LK, Pierens GK, Windley MJ, Nicholson GM, Alewood PF, King GF (2009) Direct visualization of disulfide bonds through diselenide proxies using 77Se NMR spectroscopy. Angew Chem Int Ed Engl 48:9312–9314
Muchmore DC, McIntosh LP, Russell CB, Anderson DE, Dahlquist FW (1989) Expression and nitrogen-15 labeling of proteins for proton and nitrogen-15 nuclear magnetic resonance. Methods Enzymol 177:44–73
Olejniczak ET, Dobson CM, Karplus M, Levy RM (1984) Motional averaging of proton nuclear overhauser effects in proteins. Predictions from a molecular dynamics simulation of lysozyme. J Am Chem Soc 106:1923–1930
Otting G, Liepinsh E, Wüthrich K (1993) Disulfide bond isomerization in BPTI and BPTI (G36S): an NMR study of correlated mobility in proteins. Biochemistry 32:3571–3582
Ozhogina OA, Bominaar EL (2009) Characterization of the kringle fold and identification of a ubiquitous new class of disulfide rotamers. J Struct Biol 168:223–233
Peng JW, Wagner G (1992) Mapping of spectral density functions using heteronuclear NMR relaxation measurements. J Magn Reson 98:308–322
Ramer SE, Moore RN, Vederas JC (1986) Mechanism of formation of Serine β-lactones by Mitsunobu cyclization: synthesis and use of l-Serine stereospecifically labelled with deuterium at C-3. Can J Chem 64:706
Richardson JS (1981) The anatomy and taxonomy of protein structure. Adv Prot Chem 34:167–339
Roberts RB, Abelson PH, Cowie DB, Bolton ET, Britten RJ (1957) Studies of biosynthesis in Escherichia coli, vol 607. Carnegie Institution of Washington Publications, Washington
Schmidt B, Hogg PJ (2007) Search for allosteric disulfide bonds in NMR structures. BMC Struct Biol 7:49
Schmidt B, Ho L, Hogg PJ (2006) Allosteric disulfide bonds. Biochemistry 45:7429–7433
Shaw DE, Maragakis P, Lindorff-Larsen K, Piana S, Dror RO, Eastwood MP, Bank JA, Jumper JM, Salmon JK, Shan Y, Wriggers W (2010) Atomic-level characterization of the structural dynamics of proteins. Science 330:341–346
Skalicky JJ, Mills JL, Sharma S, Szyperski T (2001) Aromatic ring-flipping in supercooled water: implications for NMR-based structural biology of proteins. J Am Chem Soc 123:388–397
Szyperski T, Luginbühl P, Otting G, Güntert P, Wüthrich K (1993) Protein dynamics studied by rotating frame 15N spin relaxation times. J Biomol NMR 3:151–164
Takahashi H, Kainosho M, Akutsu H, Fujiwara T (2010) 1H-detected 1H-1H correlation spectroscopy of a stereo-array isotope labeled amino acid under fast magic-angle spinning. J Magn Reson 203:253–256
Takeda M, Ikeya T, Güntert P, Kainosho M (2007) Automated structure determination of proteins with the SAIL-FLYA NMR method. Nat Protoc 2:2896–2902
Takeda M, Jee J, Ono AM, Terauchi T, Kainosho M (2009) Hydrogen exchange rate of tyrosine hydroxyl groups in proteins as studied by the deuterium isotope effect on Cζ chemical shifts. J Am Chem Soc 131:18556–18562
Takeda M, Terauchi T, Ono AM, Kainosho M (2010a) Application of SAIL phenylalanine and tyrosine with alternative isotope-labeling patterns for protein structure determination. J Biomol NMR 46:45–49
Takeda M, Jee J, Terauchi T, Kainosho M (2010b) Detection of the sulfhydryl groups in proteins with slow hydrogen exchange rates and determination of their proton/deuteron fractionation factors using the deuterium-induced effects on the 13Cβ NMR signals. J Am Chem Soc 132:6254–6260
Takeda M, Jee J, Ono AM, Terauchi T, Kainosho M (2011) Hydrogen exchange study on the hydroxyl groups of Serine and Threonine residues in proteins and structure refinement using NOE restraints with polar side-chain groups. J Am Chem Soc 133:17420–17427
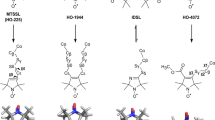
Terauchi T, Kobayashi K, Okuma K, Oba M, Nishiyama K, Kainosho M (2008) Stereoselective synthesis of triply isotope-labeled Ser, Cys, and Ala: amino acids for stereoarray isotope labeling technology. Org Lett 10:2785–2787
Torizawa T, Shimizu M, Taoka M, Miyano H, Kainosho M (2004) Efficient production of isotopically labeled proteins by cell-free synthesis: a practical protocol. J Biomol NMR 30:311–325
Vögeli B, Segawa TF, Leitz D, Sobol A, Choutko A, Trzesniak D, van Gunsteren W, Riek R (2009) Exact distances and internal dynamics of perdeuterated ubiquitin from NOE buildups. J Am Chem Soc 131:17215–17225
Vögeli B, Friedmann M, Leitz D, Sobol A, Riek R (2010) Quantitative determination of NOE rates in perdeuterated and protonated proteins: practical and theoretical aspects. J Magn Reson 204:290–302
Wagner G (1983) Characterization of the distribution of internal motions in the basic pancreatic trypsin inhibitor using a large number of internal NMR probes. Q Rev Biophys 16:1–57
Walewska A, Skalicky JJ, Davis DR, Zhang MM, Lopez-Vera E, Watkins M, Han TS, Yoshikami D, Olivera BM, Bulaj G (2008) NMR-based mapping of disulfide bridges in cysteine-rich peptides: application to the mu-conotoxin SxIIIA. J Am Chem Soc 130:14280–14286
Waugh DS (1996) Genetic tools for selective labeling of proteins with alpha-15N-amino acids. J Biomol NMR 8:148–192
Wlodawer A, Walter J, Huber R, Sjölin L (1984) Structure of bovine pancreatic trypsin inhibitor. Results of joint neutron and X-ray refinement of crystal form II. J Mol Biol 180:301–329
Wu J, Fan J, Pascal SM, Yang D (2004) General method for suppression of diagonal peaks in heteronuclear-edited NOESY spectroscopy. J Am Chem Soc 126:15018–15019
Acknowledgments
This work was supported by the Targeted Proteins Research Program (TPRP) to M. K. and partially by Grants-in-Aid for Young Scientists (B) (21770110, 23770109) to M. T. and a Grant-in-Aid in Innovative Areas (4104) to M. K.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10858_2011_9587_MOESM1_ESM.doc
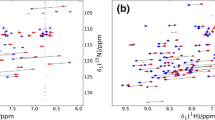
NOESY spectra (the region of the Cys5-Cys55 across-disulfide NOEs) of the UL-Cys-labeled BPTI, the l-[β-13C; α,β 2/β 3-2H2] Cys-labeled BPTI and the (l-[β-13C; α,β 2/β 3-2H2] Cys, [U-2H])-labeled BPTI at NOE mixing times of 50 and 400 ms and the comparison of their NOE build-up curves. The 1H T1 of β-protons of the Cys-labeled BPTI and the l-[β-13C; α,β 2/β 3-2H2] Cys-labeled protein in a deuterated background. The computation of inter-proton distances between Cys β-protons across a disulfide bond. The T1 and T2 values of Cys 13Cβ atoms in (l-[β-13C; α,β 2/β 3-2H2] Cys, [U-2H])-labeled BPTI. The cross-relaxation rates of the across-disulfide NOEs and the corresponding effective distances. These are provided in the supporting information. Supplementary material 1 (DOC 522 kb)
Rights and permissions
About this article
Cite this article
Takeda, M., Terauchi, T. & Kainosho, M. Conformational analysis by quantitative NOE measurements of the β-proton pairs across individual disulfide bonds in proteins. J Biomol NMR 52, 127–139 (2012). https://doi.org/10.1007/s10858-011-9587-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-011-9587-0