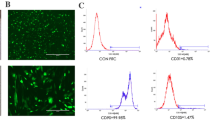
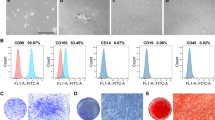
Abstract
As traditional root canal obturation leads to the loss of the biological activity of the tooth, it is necessary to develop a material that promotes the regeneration of dental tissue. However, this remains a challenging task. Our study aims to construct a mineralized material to support the proliferation and differentiation of dental pulp stem cells (DPSCs), and to explore a new strategy for the treatment of pulp tissue necrosis. Mineralized keratin (M-keratin), defined as keratin that has been mineralized in simulated body fluid, was first harvested to construct the root canal filling material. Characterizations indicated that new substances or components were formed on the surface of keratin particles after mineralization, and the morphology of the keratin was changed. M-keratin promoted the growth, proliferation, and differentiation of DPSCs. After cultivation with M-keratin, DPSCs exhibited more extracellular matrix proteins interacting with the culture interface, the number of these cells increased significantly, and the 3-[4,5-dimethylthiazol-2-yl-]-2,5-diphenyltetrazolium bromide values of cells in the experimental group also increased. Meanwhile, signs that the DPSCs began to differentiate into odontoblasts were observed or detected by alizarin red S staining, ELISA, RNA-Seq, and western blot. We hope that this study will contribute to the development of a new material that promotes the regeneration of dental tissue as well as providing new ideas and strategies for the treatment of dental pulp disease.







Similar content being viewed by others
References
Forien JB, Fleck C, Cloetens P, Duda G, Fratzl P, Zolotoyabko E, et al. Compressive residual strains in mineral nanoparticles as a possible origin of enhanced crack resistance in human tooth dentin. Nano Lett. 2015;15:3729–34.
Wang Y, Zhang T, Kwiatkowski N, Abraham BJ, Lee TI, Xie S, et al. CDK7-dependent transcriptional addiction in triple-negative breast cancer. Cell. 2015;163:174–86. https://doi.org/10.1016/j.cell.2015.08.063.
Itoh Y, Sasaki JI, Hashimoto M, Katata C, Hayashi M, Imazato S. Pulp regeneration by 3-dimensional dental pulp stem cell constructs. J Dent Res. 2018;97:1137–43.
Fioretti F, Mendoza-Palomares C, Helms M, Alam DA, Richert L, Arntz Y, et al. Nanostructured assemblies for dental application. ACS Nano. 2010;4:3277–87.
Cheng G, Li ZB. The root canal system: a channel through which we can seed cells into grafts. Med Sci Monit. 2014;20:624–7. http://www.medscimonit.com/abstract/index/idArt/890057.
Simmer JP, Papagerakis P, Smith CE, Fisher DC, Rountrey AN, Zheng L, et al. Regulation of dental enamel shape and hardness. J Dent Res. 2010;89:1024–38.
He P, Zhang Y, Kim SO, Radlanski RJ, Butcher K, Schneider RA, et al. Ameloblast differentiation in the human developing tooth: effects of extracellular matrices. Matrix Biol. 2012;29:411–9.
Zhu N, Chatzistavrou X, Ge L, Qin M, Papagerakis P, Wang Y. Biological properties of modified bioactive glass on dental pulp cells. J Dent. 2019;83:18–26. https://linkinghub.elsevier.com/retrieve/pii/S0300571218304287.
Li D, Fu L, Zhang Y, Yu Q, Ma F, Wang Z, et al. The effects of LPS on adhesion and migration of human dental pulp stem cells in vitro. J Dent. 2014;42:1327–34.
Zhou YZ, Cao Y, Liu W, Chu CH, Li QL. Polydopamine-induced tooth remineralization. ACS Appl Mater Interfaces. 2012;4:6901–10.
Collart-Dutilleul PY, Secret E, Panayotov I, Deville De Périère D, Martín-Palma RJ, Torres-Costa V, et al. Adhesion and proliferation of human mesenchymal stem cells from dental pulp on porous silicon scaffolds. ACS Appl Mater Interfaces. 2014;6:1719–28.
Rahman SU, Oh JH, Cho YD, Chung SH, Lee G, Baek JH, et al. Fibrous topography-potentiated canonical wnt signaling directs the odontoblastic differentiation of dental pulp-derived stem cells. ACS Appl Mater Interfaces. 2018;10:17526–41.
Nathanael AJ, Oyane A, Nakamura M, Sakamaki I, Nishida E, Kanemoto Y, et al. In vitro and in vivo analysis of mineralized collagen-based sponges prepared by a plasma- and precursor-assisted biomimetic process. ACS Appl Mater Interfaces. 2017;9:22185–94.
Dolatshahi-Pirouz A, Jensen T, Kraft DC, Foss M, Kingshott P, Hansen JL, et al. Fibronectin adsorption, cell adhesion, and proliferation on nanostructured tantalum surfaces. ACS Nano. 2010;4:2874–82.
Yu M, Lin Y, Liu Y, Zhou Y, Liu C, Dong L, et al. Enhanced osteointegration of hierarchical structured 3D-printed titanium implants. ACS Appl Bio Mater. 2018;1:90–9. https://doi.org/10.1021/acsabm.8b00017.
Chen G, Chen J, Yang B, Li L, Luo X, Zhang X, et al. Combination of aligned PLGA/Gelatin electrospun sheets, native dental pulp extracellular matrix and treated dentin matrix as substrates for tooth root regeneration. Biomaterials. 2015;52:56–70. https://doi.org/10.1016/j.biomaterials.2015.02.011.
Kim CS, Kim JH, Kim B, Park YS, Kim HK, Tran HT, et al. A specific groove pattern can effectively induce osteoblast differentiation. Adv Funct Mater. 2017;27:1–12.
Tu S, Zheng J, Gao X, Guan C, Cai B, Xiang L. The role of Foxq1 in proliferation of human dental pulp stem cell. Biochem Biophys Res Commun. 2018;497:543–9. https://doi.org/10.1016/j.bbrc.2018.02.077.
Han B, Xia W, Liu K, Tian F, Chen Y, Wang X, et al. Janus nanoparticles for improved dentin bonding. ACS Appl Mater Interfaces. 2018;10:8519–26.
Geuli O, Metoki N, Eliaz N, Mandler D. Electrochemically driven hydroxyapatite nanoparticles coating of medical implants. Adv Funct Mater. 2016;26:8003–10.
Chen B, Sun HH, Wang HG, Kong H, Chen FM, Yu Q. The effects of human platelet lysate on dental pulp stem cells derived from impacted human third molars. Biomaterials. 2012;33:5023–35. https://doi.org/10.1016/j.biomaterials.2012.03.057.
Türkkan S, Pazarçeviren AE, Keskin D, Machin NE, Duygulu Ö, Tezcaner A. Nanosized CaP-silk fibroin-PCL-PEG-PCL/PCL based bilayer membranes for guided bone regeneration. Mater Sci Eng C. 2017;80:484–93.
Kim YY, Ribeiro L, Maillot F, Ward O, Eichhorn SJ, Meldrum FC. Bio-inspired synthesis and mechanical properties of calcite-polymer particle composites. Adv Mater. 2010;22:2082–6.
He H, Feng M, Hu J, Chen C, Wang J, Wang X, et al. Designed short RGD peptides for one-pot aqueous synthesis of integrin-binding CdTe and CdZnTe quantum dots. ACS Appl Mater Interfaces. 2012;4:6362–70.
Das SK, Liang J, Schmidt M, Laffir F, Marsili E. Biomineralization mechanism of gold by zygomycete fungi rhizopous oryzae. ACS Nano. 2012;6:6165–73.
Veis A. A window on biomineralization. Science. 2005;307:1419–20.
Zhao X, Lui YS, Choo CKC, Sow WT, Huang CL, Ng KW, et al. Calcium phosphate coated keratin-PCL scaffolds for potential bone tissue regeneration. Mater Sci Eng C. 2015;49:746–53.
Guzman RC, de, Justin MS, Mary DE, Michelle RM, Heather BC, Thomas LS, et al. Bone regeneration with BMP-2 delivered from keratose scaffolds. Biomaterials. 2013;34:1644–56. https://doi.org/10.1016/j.biomaterials.2012.11.002.
Xu S, Sang L, Zhang Y, Wang X, Li X. Biological evaluation of human hair keratin scaffolds for skin wound repair and regeneration. Mater Sci Eng C. 2013;33:648–55. https://doi.org/10.1016/j.msec.2012.10.011.
Yoon KH, Yoon M, Moir RD, Khuon S, Flitney FW, Goldman RD. Insights into the dynamic properties of keratin intermediate filaments in living epithelial cells. J Cell Biol. 2001;152:503–16.
Kim HJ, Choi WJ, Lee CH. Phosphorylation and reorganization of keratin networks: Implications for carcinogenesis and epithelial mesenchymal transition. Biomol Ther. 2015;23:301–12.
Sharma LA, Love RM, Ali MA, Sharma A, Macari S, Avadhani A, et al. Healing response of rat pulp treated with an injectable keratin hydrogel. J Appl Biomater Funct Mater. 2017;15:e244–50.
Ajay Sharma L, Ali MA, Love RM, Wilson MJ, Dias GJ. Novel keratin preparation supports growth and differentiation of odontoblast-like cells. Int Endod J. 2016;49:471–82.
Bakopoulou A, Papachristou E, Bousnaki M, Hadjichristou C, Kontonasaki E, Theocharidou A, et al. Human treated dentin matrices combined with Zn-doped, Mg-based bioceramic scaffolds and human dental pulp stem cells towards targeted dentin regeneration. Dent Mater. 2016;32:e159–75. https://doi.org/10.1016/j.dental.2016.05.013.
Rapino M, Di Valerio V, Zara S, Gallorini M, Marconi GD, Sancilio S, et al. Chitlac-coated thermosets enhance osteogenesis and angiogenesis in a co-culture of dental pulp stem cells and endothelial cells. Nanomaterials. 2019;9:928–40.
Rivero G, Aldana AA, Frontini Lopez YR, Liverani L, Boccacini AR, Bustos DM, et al. 14-3-3ε protein-immobilized PCL-HA electrospun scaffolds with enhanced osteogenicity. J Mater Sci Mater Med. 2019;30:2–5. https://doi.org/10.1007/s10856-019-6302-2.
Ahmed HH, Aglan HA, Mabrouk M, Abd-Rabou AA, Beherei HH. Enhanced mesenchymal stem cell proliferation through complexation of selenium/titanium nanocomposites. J Mater Sci Mater Med. 2019;30. https://doi.org/10.1007/s10856-019-6224-z.
Matsui M, Kobayashi T, Tsutsui TW. CD146 positive human dental pulp stem cells promote regeneration of dentin/pulp-like structures. Hum Cell. 2018;31:127–38. https://doi.org/10.1007/s13577-017-0198-2.
Yin L, Yang S, He M, Chang Y, Wang K, Zhu Y, et al. Physicochemical and biological characteristics of BMP-2/IGF-1-loaded three-dimensional coaxial electrospun fibrous membranes for bone defect repair. J Mater Sci Mater Med. 2017;28:1–14. https://doi.org/10.1007/s10856-017-5898-3.
Giraldez MD, Spengler RM, Etheridge A, Goicochea AJ, Tuck M, Choi SW, et al. Phospho‐RNA‐seq: a modified small RNA‐seq method that reveals circulating mRNA and lncRNA fragments as potential biomarkers in human plasma. EMBO J. 2019;38:1–14.
Luo Z, Li D, Kohli MR, Yu Q, Kim S, He WX. Effect of BiodentineTM on the proliferation, migration and adhesion of human dental pulp stem cells. J Dent. 2014;42:490–7.
Tammaro L, Vittoria V, Calarco A, Petillo O, Riccitiello F, Peluso G. Effect of layered double hydroxide intercalated with fluoride ions on the physical, biological and release properties of a dental composite resin. J Dent. 2014;42:60–7.
Zhang Y, Zhang ZN, Li N, Zhao LJ, Xue Y, Wu HJ, et al. Nbr1-regulated autophagy in Lactoferrin-induced osteoblastic differentiation. Biosci Biotechnol Biochem. 2020;84:1191–200.
Zhou ZF, Sun TW, Chen F, Zuo DQ, Wang HS, Hua YQ, et al. Calcium phosphate-phosphorylated adenosine hybrid microspheres for anti-osteosarcoma drug delivery and osteogenic differentiation. Biomaterials. 2017;121:1–14. https://doi.org/10.1016/j.biomaterials.2016.12.031.
Mousavi SJ, Doweidar MH. Numerical modeling of cell differentiation and proliferation in force-induced substrates via encapsulated magnetic nanoparticles. Comput Methods Programs Biomed. 2016;130:106–17.
He L, Si G, Huang J, Samuel ADT, Perrimon N. Mechanical regulation of stem-cell differentiation by the stretch-activated Piezo channel. Nature. 2018;555:103–6.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51672276), Guangdong Basic and Applied Basic Research Foundation (2019A1515110747), and College Students’ Innovative Entrepreneurial Training Program (S201911847083).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, WY., Li, X., Feng, Y. et al. M-keratin nano-materials create a mineralized micro-circumstance to promote proliferation and differentiation of DPSCs. J Mater Sci: Mater Med 31, 124 (2020). https://doi.org/10.1007/s10856-020-06465-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-020-06465-8