Abstract
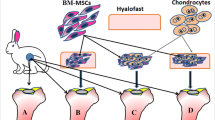
Bone marrow mesenchymal stem cells (BMSCs) are well-known for tissue regeneration and bone repair. This study intended to evaluate the potential efficiency BMSCs in poly(lactide-co-glycolide) (PLGA) scaffolds for the treatment of laryngeal cartilage defects. BMSCs were isolated and identified, and added with 10 ng/mL transforming growth factor-beta1 (TGF-β1) or/and 300 ng/mL CDMP1 to coculture with PLGA scaffolds. The chondrogenic differentiation, migration, and apoptosis of BMSCs were detected under the action of TGF-β1 or/and CDMP1. After successful modeling of laryngeal cartilage defects, PLGA scaffolds were transplanted into the rabbits correspondingly. After 8 weeks, laryngeal cartilage defects were assessed. Levels of collagen II, aggrecan, Sox9, Smad2, Smad3, ERK, and JNK were detected. The TGF-β1 or/and CDMP1-induced BMSCs expressed collagen II, aggrecan, and Sox9, with enhanced cell migration and inhibited apoptosis. In addition, laryngeal cartilage defect in rabbits with TGF-β1 or/and CDMP1 was alleviated, and levels of specific cartilage matrix markers were decreased. The combined effects of TGF-β1 and CDMP1 were more significant. The TGF-β1/Smad and ERK/JNK pathways were activated after TGF-β1 or/and CDMP1 were added to BMSCs or rabbits. In summary, BMSCs and PLGA scaffolds repair laryngeal cartilage defects in rabbits by activating the TGF-β1/Smad and ERK/JNK pathways under the coaction of TGF-β1 and CDMP1.







Similar content being viewed by others
References
Chen S, Fu P, Wu H, Pei M. Meniscus, articular cartilage and nucleus pulposus: a comparative review of cartilage-like tissues in anatomy, development and function. Cell Tissue Res. 2017;370:53–70. https://doi.org/10.1007/s00441-017-2613-0.
Ming L, Zhipeng Y, Fei Y, Feng R, Jian W, Baoguo J, et al. Microfluidic-based screening of resveratrol and drug-loading PLA/Gelatine nano-scaffold for the repair of cartilage defect. Artif Cells Nanomed Biotechnol. 2018;46:336–46. https://doi.org/10.1080/21691401.2017.1423498.
Lubis AM, Sandhow L, Lubis VK, Noor A, Gumay F, Merlina M, et al. Isolation and cultivation of mesenchymal stem cells from iliac crest bone marrow for further cartilage defect management. Acta Med Indones. 2011;43:178–84.
Roffi A, Krishnakumar GS, Gostynska N, Kon E, Candrian C, Filardo G. The role of three-dimensional scaffolds in treating long bone defects: evidence from preclinical and clinical literature-a systematic review. Biomed Res Int. 2017;2017:8074178. https://doi.org/10.1155/2017/8074178.
Majidinia M, Sadeghpour A, Yousefi B. The roles of signaling pathways in bone repair and regeneration. J Cell Physiol. 2018;233:2937–48. https://doi.org/10.1002/jcp.26042.
Lee WY, Wang B. Cartilage repair by mesenchymal stem cells: clinical trial update and perspectives. J Orthop Transl. 2017;9:76–88. https://doi.org/10.1016/j.jot.2017.03.005.
Richardson SM, Kalamegam G, Pushparaj PN, Matta C, Memic A, Khademhosseini A, et al. Mesenchymal stem cells in regenerative medicine: focus on articular cartilage and intervertebral disc regeneration. Methods 2016;99:69–80. https://doi.org/10.1016/j.ymeth.2015.09.015.
Shao J, Zhang W, Yang T. Using mesenchymal stem cells as a therapy for bone regeneration and repairing. Biol Res. 2015;48:62. https://doi.org/10.1186/s40659-015-0053-4.
Benders KE, van Weeren PR, Badylak SF, Saris DB, Dhert WJ, Malda J. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol. 2013;31:169–76. https://doi.org/10.1016/j.tibtech.2012.12.004.
Li JW, Guo XL, He CL, Tuo YH, Wang Z, Wen J, et al. In vitro chondrogenesis of the goat bone marrow mesenchymal stem cells directed by chondrocytes in monolayer and 3-dimetional indirect co-culture system. Chin Med J. 2011;124:3080–6.
Moser C, Bardsley K, El Haj AJ, Alini M, Stoddart MJ, Bara JJ. A perfusion culture system for assessing bone marrow stromal cell differentiation on PLGA Scaffolds for bone repair. Front Bioeng Biotechnol. 2018;6:161. https://doi.org/10.3389/fbioe.2018.00161.
Wu G, Cui Y, Wang YT, Yao M, Hu J, Li JX, et al. Repair of cartilage defects in BMSCs via CDMP1 gene transfection. Genet Mol Res. 2014;13:291–301. https://doi.org/10.4238/2014.January.17.14.
Zatroch KK, Knight CG, Reimer JN, Pang DS. Refinement of intraperitoneal injection of sodium pentobarbital for euthanasia in laboratory rats (Rattus norvegicus). BMC Vet Res. 2017;13:60. https://doi.org/10.1186/s12917-017-0982-y.
Zhang H, Voytik-Harbin S, Brookes S, Zhang L, Wallace J, Parker N, et al. Use of autologous adipose-derived mesenchymal stem cells for creation of laryngeal cartilage. Laryngoscope 2018;128:E123–E9. https://doi.org/10.1002/lary.26980.
Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJ, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117:889–97. https://doi.org/10.1242/jcs.00912.
Sobue Y, Kojima T, Kurokouchi K, Takahashi S, Yoshida H, Poole R, et al. Prediction of progression of damage to articular cartilage 2 years after anterior cruciate ligament reconstruction: use of aggrecan and type II collagen biomarkers in a retrospective observational study. Arthritis Res Ther. 2017;19:265. https://doi.org/10.1186/s13075-017-1471-1.
Hattori S, Oxford C, Reddi AH. Identification of superficial zone articular chondrocyte stem/progenitor cells. Biochem Biophys Res Commun. 2007;358:99–103. https://doi.org/10.1016/j.bbrc.2007.04.142.
Muinos-Lopez E, Rendal-Vazquez ME, Hermida-Gomez T, Fuentes-Boquete I, Diaz-Prado S, Blanco FJ. Cryopreservation effect on proliferative and chondrogenic potential of human chondrocytes isolated from superficial and deep cartilage. Open Orthop J 2012;6:150–9. https://doi.org/10.2174/1874325001206010150.
Gomez-Leduc T, Hervieu M, Legendre F, Bouyoucef M, Gruchy N, Poulain L, et al. Chondrogenic commitment of human umbilical cord blood-derived mesenchymal stem cells in collagen matrices for cartilage engineering. Sci Rep. 2016;6:32786. https://doi.org/10.1038/srep32786.
Liu S, Jia Y, Yuan M, Guo W, Huang J, Zhao B, et al. Repair of osteochondral defects using human umbilical cord Wharton’s jelly-derived mesenchymal stem cells in a rabbit model. Biomed Res Int. 2017;2017:8760383. https://doi.org/10.1155/2017/8760383.
Lo Monaco M, Merckx G, Ratajczak J, Gervois P, Hilkens P, Clegg P, et al. Stem cells for cartilage repair: preclinical studies and insights in translational animal models and outcome measures. Stem Cells Int. 2018;2018:9079538. https://doi.org/10.1155/2018/9079538.
Caminal M, Moll X, Codina D, Rabanal RM, Morist A, Barrachina J, et al. Transitory improvement of articular cartilage characteristics after implantation of polylactide:polyglycolic acid (PLGA) scaffolds seeded with autologous mesenchymal stromal cells in a sheep model of critical-sized chondral defect. Biotechnol Lett. 2014;36:2143–53. https://doi.org/10.1007/s10529-014-1585-3.
Tang Y, Xiao J, Wang Y, Li M, Shi Z. Effect of adenovirus-mediated TGF-beta1 gene transfer on the function of rabbit articular chondrocytes. J Orthop Sci. 2017;22:149–55. https://doi.org/10.1016/j.jos.2016.05.009.
Yin F, Cai J, Zen W, Wei Y, Zhou W, Yuan F, et al. Cartilage regeneration of adipose-derived stem cells in the TGF-beta1-immobilized PLGA-gelatin scaffold. Stem Cell Rev Rep. 2015;11:453–9. https://doi.org/10.1007/s12015-014-9561-9.
Cui Y, Yao M, Liu Y, Mu L, Zhang B, Wu G. Effects of cartilage-derived morphogenetic protein 1 (CDMP1) transgenic mesenchymal stem cell sheets in repairing rabbit cartilage defects. Genet Mol Res. 2016;15. https://doi.org/10.4238/gmr.15028058.
Katayama R, Wakitani S, Tsumaki N, Morita Y, Matsushita I, Gejo R, et al. Repair of articular cartilage defects in rabbits using CDMP1 gene-transfected autologous mesenchymal cells derived from bone marrow. Rheumatology. 2004;43:980–5. https://doi.org/10.1093/rheumatology/keh240.
Zhu X, Gao Q, Zhao G, Wang H, Liu L, Chen Z, et al. Comparison study of bone defect healing effect of raw and processed pyritum in rats. Biol Trace Elem Res. 2018;184:136–47. https://doi.org/10.1007/s12011-017-1166-0.
He CX, Zhang TY, Miao PH, Hu ZJ, Han M, Tabata Y, et al. TGF-beta1 gene-engineered mesenchymal stem cells induce rat cartilage regeneration using nonviral gene vector. Biotechnol Appl Biochem. 2012;59:163–9. https://doi.org/10.1002/bab.1001.
Thielen NGM, van der Kraan PM, van Caam APM. TGFbeta/BMP Signaling pathway in cartilage homeostasis. Cells. 2019;8. https://doi.org/10.3390/cells8090969.
Finnson KW, Chi Y, Bou-Gharios G, Leask A, Philip A. TGF-b signaling in cartilage homeostasis and osteoarthritis. Front Biosci. 2012;4:251–68.
Blaney Davidson EN, Vitters EL, van der Kraan PM, van den Berg WB. Expression of transforming growth factor-beta (TGFbeta) and the TGFbeta signalling molecule SMAD-2P in spontaneous and instability-induced osteoarthritis: role in cartilage degradation, chondrogenesis and osteophyte formation. Ann Rheum Dis. 2006;65:1414–21. https://doi.org/10.1136/ard.2005.045971.
Li TF, Darowish M, Zuscik MJ, Chen D, Schwarz EM, Rosier RN, et al. Smad3-deficient chondrocytes have enhanced BMP signaling and accelerated differentiation. J Bone Min Res. 2006;21:4–16. https://doi.org/10.1359/JBMR.050911.
Jiang X, Huang B, Yang H, Li G, Zhang C, Yang G, et al. TGF-beta1 is involved in vitamin D-induced chondrogenic differentiation of bone marrow-derived mesenchymal stem cells by regulating the ERK/JNK pathway. Cell Physiol Biochem. 2017;42:2230–41. https://doi.org/10.1159/000479997.
Ma N, Teng X, Zheng Q, Chen P. The regulatory mechanism of p38/MAPK in the chondrogenic differentiation from bone marrow mesenchymal stem cells. J Orthop Surg Res. 2019;14:434. https://doi.org/10.1186/s13018-019-1505-2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
This study was approved and supervised by the animal ethics committee of Affiliated Hospital of Jining Medical University. All procedures were strictly conducted in line with the Code of Ethics. Significant efforts were made to minimize the number of animals and their respective suffering.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, L., Zhang, Y. & Wang, C. Coaction of TGF-β1 and CDMP1 in BMSCs-induced laryngeal cartilage repair in rabbits. J Mater Sci: Mater Med 31, 130 (2020). https://doi.org/10.1007/s10856-020-06454-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-020-06454-x