Abstract
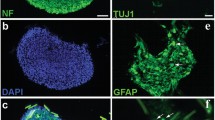
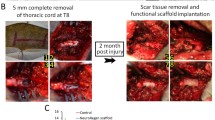
Persistent local oxygen delivery is crucial to create a microenvironment for cell survival and nerve regeneration in acute spinal cord injury (SCI). This study aimed to fabricate calcium peroxide-based microspheres incorporated into a 3-D construct scaffold as a novel oxygen release therapy for SCI. The scaffolds were able to generate oxygen over the course of 21 days when incubated under hypoxic conditions. In vitro, GFP-labeled bone marrow-derived mesenchymal stem cells (MSCs) were planted into the scaffolds. We observed that scaffolds could enhance MSC survival under hypoxic conditions for more than 21 days. Oxygen generating scaffolds were transplanted into spinal cord injury sites of rats in vivo. Twelve weeks following transplantation, cavity areas in the injury/graft site were significantly reduced due to tissue regeneration. Additionally, the oxygen generating scaffolds improved revascularization as observed through vWF immunostaining. A striking feature was the occurrence of nerve fiber regeneration in the lesion sites, which eventually led to significant locomotion recovery. The present results indicate that the oxygen generating scaffolds have the property of sustained local oxygen release, thus facilitating regeneration in injured spinal cords.










Similar content being viewed by others
References
Jain NB, Ayers GD, Peterson EN, Harris MB, Morse L, O'Connor KC, et al. Traumatic spinal cord injury in the United States, 1993-2012. JAMA. 2015;313:2236–43.
Silva NA, Sousa N, Reis RL, Salgado AJ. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol. 2014;114:25–57.
Anwar MA, Al Shehabi TS, Eid AH. Inflammogenesis of secondary spinal cord injury. Front Cell Neurosci. 2016;10:98.
Huang WL, George KJ, Ibba V, Liu MC, Averill S, Quartu M, et al. The characteristics of neuronal injury in a static compression model of spinal cord injury in adult rats. Eur J Neurosci. 2007;25:362–72.
Li X, Zhao Y, Cheng S, Han S, Shu M, Chen B, et al. Cetuximab modified collagen scaffold directs neurogenesis of injury-activated endogenous neural stem cells for acute spinal cord injury repair. Biomaterials. 2017;137:73–86.
Li G, Che MT, Zhang K, Qin LN, Zhang YT, Chen RQ, et al. Graft of the NT-3 persistent delivery gelatin sponge scaffold promotes axon regeneration, attenuates inflammation, and induces cell migration in rat and canine with spinal cord injury. Biomaterials. 2016;83:233–48.
Fan C, Li X, Xiao Z, Zhao Y, Liang H, Wang B, et al. A modified collagen scaffold facilitates endogenous neurogenesis for acute spinal cord injury repair. Acta Biomater. 2017;51:304–16.
Han S, Wang B, Jin W, Xiao Z, Li X, Ding W, et al. The linear-ordered collagen scaffold-BDNF complex significantly promotes functional recovery after completely transected spinal cord injury in canine. Biomaterials. 2015;41:89–96.
Papandreou I, Powell A, Lim AL, Denko N. Cellular reaction to hypoxia: sensing and responding to an adverse environment. Mutat Res. 2005;569:87–100.
Gross JD, Long RC Jr, Constantinidis I, Sambanis A. Monitoring of dissolved oxygen and cellular bioenergetics within a pancreatic substitute. Biotechnol Bioeng. 2007;98:261–70.
Zhu S, Ge J, Wang Y, Qi F, Ma T, Wang M, et al. A synthetic oxygen carrier-olfactory ensheathing cell composition system for the promotion of sciatic nerve regeneration. Biomaterials. 2014;35:1450–61.
Ma T, Wang Y, Qi F, Zhu S, Huang L, Liu Z, et al. The effect of synthetic oxygen carrier-enriched fibrin hydrogel on Schwann cells under hypoxia condition in vitro. Biomaterials. 2013;34:10016–27.
Sun L, Zhao L, Li P, Liu X, Liang F, Jiang Y, et al. Effect of hyperbaric oxygen therapy on HMGB1/NF-κB expression and prognosis of acute spinal cord injury: a randomized clinical trial. Neurosci Lett. 2019;692:47–52.
Patel NP, Huang JH. Hyperbaric Oxyg Ther spinal cord Inj. 2017;7:133–43.
Sanders RW, Katz KD, Suyama J, Akhtar J, O'Toole KS, Corll D, et al. Seizure during hyperbaric oxygen therapy for carbon monoxide toxicity: a case series and five-year experience. J Emerg Med. 2012;42:e69–72.
Abdi SI, Ng SM, Lim JO. An enzyme-modulated oxygen-producing micro-system for regenerative therapeutics. Int J Pharm. 2011;409:203–5.
Oh SH, Ward CL, Atala A, Yoo JJ, Harrison BS. Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials. 2009;30:757–62.
Ng SM, Choi JY, Han HS, Huh JS, Lim JO. Novel microencapsulation of potential drugs with low molecular weight and high hydrophilicity: hydrogen peroxide as a candidate compound. Int J Pharm. 2010;384:120–7.
Li Z, Guo X, Guan J. An oxygen release system to augment cardiac progenitor cell survival and differentiation under hypoxic condition. Biomaterials. 2012;33:5914–23.
Nguyen LH, Gao M, Lin J, Wu W, Wang J, Chew SY. Three-dimensional aligned nanofibers-hydrogel scaffold for controlled non-viral drug/gene delivery to direct axon regeneration in spinal cord injury treatment. Sci Rep. 2017;7:42212.
Rong Z-J, Yang L-J, Cai B-T, Zhu L-X, Cao Y-L, Wu G-F, et al. Porous nano-hydroxyapatite/collagen scaffold containing drug-loaded ADM–PLGA microspheres for bone cancer treatment. J Mater Sci: Mater Med. 2016;27:89.
Wan JM, Liu LL, Zhang JF, Lu JW, Li Q. Promotion of neuronal regeneration by using self-polymerized dendritic polypeptide scaffold for spinal cord tissue engineering. J Mater Sci Mater Med. 2017;29:6.
Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc Natl Acad Sci. 2012;109:4245–50.
Wu G, Pan M, Wang X, Wen J, Cao S, Li Z, et al. Osteogenesis of peripheral blood mesenchymal stem cells in self assembling peptide nanofiber for healing critical size calvarial bony defect. Sci Rep. 2015;5:16681.
Webb AA, Jeffery ND, Olby NJ, Muir GD. Behavioural analysis of the efficacy of treatments for injuries to the spinal cord in animals. Vet Rec. 2004;155:225–30.
Starke RD, Ferraro F, Paschalaki KE, Dryden NH, McKinnon TA, Sutton RE, et al. Endothelial von Willebrand factor regulates angiogenesis. Blood. 2011;117:1071–80.
Snider S, Cavalli A, Colombo F, Gallotti AL, Quattrini A, Salvatore L, et al. A novel composite type I collagen scaffold with micropatterned porosity regulates the entrance of phagocytes in a severe model of spinal cord injury. J Biomed Mater Res B Appl Biomater. 2017;105:1040–53.
Park JH, Min J, Baek SR, Kim SW, Kwon IK, Jeon SR. Enhanced neuroregenerative effects by scaffold for the treatment of a rat spinal cord injury with Wnt3a-secreting fibroblasts. Acta Neurochir. 2013;155:809–16.
Cigognini D, Satta A, Colleoni B, Silva D, Donegà M, Antonini S, et al. Evaluation of early and late effects into the acute spinal cord injury of an injectable functionalized self-assembling scaffold. PLoS ONE. 2011;6:e19782.
Zhang W, Smith C, Howlett C, Stanimirovic D. Inflammatory activation of human brain endothelial cells by hypoxic astrocytes in vitro is mediated by IL-1beta. J Cereb Blood Flow Metab. 2000;20:967–78.
Benjamin S, Sheyn D, Ben-David S, Oh A, Kallai I, Li N, et al. Oxygenated environment enhances both stem cell survival and osteogenic differentiation. Tissue Eng Part A. 2013;19:748–58.
Fox CJJ. On the coefficients of absorption of nitrogen and oxygen in distilled water and sea-water, and of atmospheric carbonic acid in sea-water. Trans Faraday Soc. 1909;5:68–86.
Wang Y, Qi F, Zhu S, Ye Z, Ma T, Hu X, et al. A synthetic oxygen carrier in fibrin matrices promotes sciatic nerve regeneration in rats. Acta Biomater. 2013;9:7248–63.
Varde NK, Pack DW. Influence of particle size and antacid on release and stability of plasmid DNA from uniform PLGA microspheres. J Control Release. 2007;124:172–80.
Ma G. Microencapsulation of protein drugs for drug delivery: strategy, preparation, and applications. J Control Release. 2014;193:324–40.
Algul D, Sipahi H, Aydin A, Kelleci F, Ozdatli S, Yener FG. Biocompatibility of biomimetic multilayered alginate-chitosan/beta-TCP scaffold for osteochondral tissue. Int J Biol Macromol. 2015;79:363–9.
Gao J, Wang M, Shi C, Wang L, Wang D, Zhu Y. Synthesis of trace element Si and Sr codoping hydroxyapatite with non-cytotoxicity and enhanced cell proliferation and differentiation. Biol Trace Elem Res. 2016;174:208–17.
Yu Y, Matsuyama Y, Yanase M, Ito S, Adachi K, Satake K, et al. Effects of hyperbaric oxygen on GDNF expression and apoptosis in spinal cord injury. Neuroreport. 2004;15:2369–73.
Susilo I, Devi A, Purwandhono A, Warsito SH. Effects of hyperbaric oxygen therapy in enhancing expressions of e-NOS, TNF-α and VEGF in wound healing. J Phys: Conf Ser. 2017;853:012030.
Wang Y, Zhang S, Luo M, Li Y. Hyperbaric oxygen therapy improves local microenvironment after spinal cord injury. Neural Regen Res. 2014;9:2182–8.
Wei L, Wang J, Cao Y, Ren Q, Zhao L, Li X, et al. Hyperbaric oxygenation promotes neural stem cell proliferation and protects the learning and memory ability in neonatal hypoxic-ischemic brain damage. Int J Clin Exp Pathol. 2015;8:1752–9.
Yu S, Yao S, Wen Y, Wang Y, Wang H, Xu Q. Angiogenic microspheres promote neural regeneration and motor function recovery after spinal cord injury in rats. Sci Rep. 2016;6:33428.
Yahata K, Kanno H, Ozawa H, Yamaya S, Tateda S, Ito K, et al. Low-energy extracorporeal shock wave therapy for promotion of vascular endothelial growth factor expression and angiogenesis and improvement of locomotor and sensory functions after spinal cord injury. J Neurosurg Spine. 2016;25:745–55.
Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002;99:11946–50.
Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–87.
Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev Cell. 2009;16:196–208.
Dray C, Rougon G, Debarbieux F. Quantitative analysis by in vivo imaging of the dynamics of vascular and axonal networks in injured mouse spinal cord. Proc Natl Acad Sci USA. 2009;106:9459–64.
McCreedy DA, Wilems TS, Xu H, Butts JC, Brown CR, Smith AW, et al. Survival, differentiation, and migration of high-purity mouse embryonic stem cell-derived progenitor motor neurons in fibrin scaffolds after sub-acute spinal cord injury. Biomater Sci. 2014;2:1672–82.
Chedly J, Soares S, Montembault A, von Boxberg Y, Veron-Ravaille M, Mouffle C, et al. Physical chitosan microhydrogels as scaffolds for spinal cord injury restoration and axon regeneration. Biomaterials. 2017;138:91–107.
Tian WM, Zhang CL, Hou SP, Yu X, Cui FZ, Xu QY, et al. Hyaluronic acid hydrogel as Nogo-66 receptor antibody delivery system for the repairing of injured rat brain: in vitro. J Control Release. 2005;102:13–22.
Breen BA, Kraskiewicz H, Ronan R, Kshiragar A, Patar A, Sargeant T, et al. Therapeutic effect of neurotrophin-3 treatment in an injectable collagen scaffold following rat spinal cord hemisection injury. ACS Biomater Sci Eng. 2017;3:1287–95.
Acknowledgements
This research was supported by National Natural Science Foundation of China (81672140, 81974329), Natural Science Foundation of Guangdong Province (2017A030313111). Wenzhou Municipal Science and Technology Project of China (No. Y20170391). The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, L., Wan, J., Dai, M. et al. Effects of oxygen generating scaffolds on cell survival and functional recovery following acute spinal cord injury in rats. J Mater Sci: Mater Med 31, 115 (2020). https://doi.org/10.1007/s10856-020-06453-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-020-06453-y