Abstract
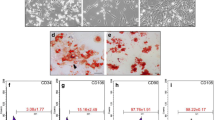
In this study, we aimed at fabricating decellularized bovine myocardial extracellular matrix-based films (dMEbF) for cardiac tissue engineering (CTE). The decellularization process was carried out utilizing four consecutive stages including hypotonic treatment, detergent treatment, enzymatic digestion and decontamination, respectively. In order to fabricate the dMEbF, dBM were digested with pepsin and gelation process was conducted. dMEbF were then crosslinked with N-hydroxysuccinimide/1-Ethyl-3-(3-dimethylaminopropyl)-carbodiimide (NHS/EDC) to increase their durability. Nuclear contents of native BM and decellularized BM (dBM) tissues were determined with DNA content analysis and agarose-gel electrophoresis. Cell viability on dMEbF for 3rd, 7th, and 14th days was assessed by MTT assay. Cell attachment on dMEbF was also studied by scanning electron microscopy. Trans-differentiation capacity of human adipose-derived mesenchymal stem cells (hAMSCs) into cardiomyocyte-like cells on dMEbF were also evaluated by histochemical and immunohistochemical analyses. DNA contents for native and dBM were, respectively, found as 886.11 ± 164.85 and 47.66 ± 0.09 ng/mg dry weight, indicating a successful decellularization process. The results of glycosaminoglycan and hydroxyproline assay, and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), performed in order to characterize the extracellular matrix (ECM) composition of native and dBM tissue, showed that the BM matrix was not damaged during the proposed method. Lastly, regarding the histological study, dMEbF not only mimics native ECM, but also induces the stem cells into cardiomyocyte-like cells phenotype which brings it the potential of use in CTE.






Similar content being viewed by others
References
Wang B, Borazjani A, Tahai M, De Jongh Curry AL, Simionescu DT, Guan J, et al. Fabrication of cardiac patch with decellularized porcine myocardial scaffold and bone marrow mononuclear cells. J Biomed Mater Res Part A. 2010;94:1100–10.
Wang RM, Christman KL. Decellularized myocardial matrix hydrogels: In basic research and preclinical studies. Adv Drug Deliv Rev. 2016;96:77–82.
Wu KH, Liu YL, Zhou B, Han ZC. Cellular therapy and myocardial tissue engineering: the role of adult stem and progenitor cells. Eur J Cardiothorac Surg. 2006;30:770–81.
Yeong WY, Sudarmadji N, Yu HY, Chua CK, Leong KF, Venkatraman SS, et al. Porous polycaprolactone scaffold for cardiac tissue engineering fabricated by selective laser sintering. Acta Biomater. 2010;6:2028–34.
Venugopal JR, Prabhakaran MP, Mukherjee S, Ravichandran R, Dan K, Ramakrishna S. Biomaterial strategies for alleviation of myocardial infarction. J R Soc Interface. 2012;9:1–19.
Weymann A, Loganathan S, Takahashi H, Schies C, Claus B, Hirschberg K, et al. Development and evaluation of a perfusion decellularization porcine heart model. Circ J. 2011;75:852–60.
Nugent HM, Edelman ER. Tissue engineering therapy for cardiovascular disease. Circ Res. 2003;92:1068–78.
Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76.
Shimizu K, Ito A, Arinobe M, Murase Y, Iwata Y, Narita Y, et al. Effective cell-seeding technique using magnetite nanoparticles and magnetic force onto decellularized blood vessels for vascular tissue engineering. J Biosci Bioeng. 2007;103:472–8.
Langer R, Vacanti JP. Tissue Engineering. Science. 1993;260:920–6.
Demirbag B, Huri PY, Kose GT, Buyuksungur A, Hasirci V. Advanced cell therapies with and without scaffolds. Biotechnol J. 2011;6:1437–53.
Arslan YE, Hiz MM, Sezgin Arslan T. The use of decellularized animal tissues in regenerative therapies. Kafkas Univ Vet Fak Derg. 2015;21:139–45.
Shin H, Jo S, Mikos AG. Biomimetic materials for tissue engineering. Biomaterials. 2003;24:4353–64.
Ma Z, Kotaki M, Inai R, Ramakrishna S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005;11:101–9.
Arslan YE, Sezgin Arslan T, Derkus B, Emregul E, Emregul KC. Fabrication of human hair keratin/jellyfish collagen/eggshell-derived hydroxyapatite osteoinductive biocomposite scaffolds for bone tissue engineering: From waste to regenerative medicine products. Colloids Surf B Biointerfaces. 2017;154:160–70.
Chen Q, Liang S, Thouas GA. Elastomeric biomaterials for tissue engineering. Prog Polym Sci. 2013;38:584–671.
Shadrin IY, Allen BW, Qian Y, Jackman CP, Carlson AL, Juhas ME, et al. Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nat Commun. 2017;8:1825.
Song JJ, Ott HC. Organ engineering based on decellularized matrix scaffolds. Trends Mol Med. 2011;17:424–32.
Pagoulatou E, Triantaphyllidou IE, Vynios DH, Papachristou DJ, Koletsis E, Deligianni D, et al. Biomechanical and structural changes following the decellularization of bovine pericardial tissues for use as a tissue engineering scaffold. J Mater Sci Mater Med. 2012;23:1387–96.
Erten E, Sezgin Arslan T, Derkus B, Arslan YE. Detergent-free decellularization of bovine costal cartilage for chondrogenic differentiation of human adipose mesenchymal stem cells in vitro. RSC Adv. 2016;6:94236–46.
Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–83.
Mendoza-Novelo B, Avila EE, Cauich-Rodríguez JV, Jorge-Herrero E, Rojo FJ, Guinea GV, Mata-Mata HL. Decellularization of pericardial tissue and its impact on tensile viscoelasticity and glycosaminoglycan content. Acta Biomater. 2011;7:1241–8.
Pourfarhangia KE, Mashayekhana S, Asla SG, Hajebrahimic Z. Construction of scaffolds composed of acellular cardiac extracellular matrix for myocardial tissue engineering. Biologicals. 2018;53:10–8.
Ye X, Wang H, Gong W, Li S, Li H, Wang Z, Zhao Q. Impact of decellularization on porcine myocardium as scaffold for tissue engineered heart tissue. J Mater Sci Mater Med. 2016;27:70
Poornejad N, Nielsen JJ, Morris RJ, Gassman JR, Reynolds PR, Roeder BL, et al. Comparison of four decontamination treatments on porcine renal decellularized extracellular matrix structure, composition, and support of human renal cortical tubular epithelium cells. J Biomater Appl. 2016;30:1154–67.
Wilshaw S-P, Kearney JN, Fisher J, Ingham E. Production of an acellular amniotic membrane matrix for use in tissue engineering. Tissue Eng. 2006;12:2117–29.
Sawkins MJ, Bowen W, Dhadda P, Markides H, Sidney LE, Taylor AJ, et al. Hydrogels derived from demineralized and decellularized bone extracellular matrix. Acta Biomater. 2013;9:7865–73.
Buttafoco L, Kolkman NG, Engbers-Buijtenhuijs P, Poot AA, Dijkstra PJ, Vermes I, et al. Electrospinning of collagen and elastin for tissue engineering applications. Biomaterials. 2006;27:724–34.
Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30:5409–16.
Derkus B, Arslan YE, Bayrac AT, Kantarcioglu I, Emregul KC, Emregul E. Development of a novel aptasensor using jellyfish collagen as matrix and thrombin detection in blood samples obtained from patients with various neurodisease. Sens Actuators B Chem. 2016;228:725–36.
Seif-Naraghi SB, Horn D, Schup-Magoffin PJ, Christman KL. Injectable extracellular matrix derived hydrogel provides a platform for enhanced retention and delivery of a heparin-binding growth factor. Acta Biomater. 2012;8:3695–703.
Pati F, Jang J, Ha DH, Won Kim S, Rhie JW, Shim JH, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014;5:1–11.
Porzionato A, Sfriso MM, Pontini A, Macchi V, Petrelli L, Pavan PG, et al. Decellularized human skeletal muscle as biologic scaffold for reconstructive surgery. Int J Mol Sci. 2015;16:14808–31.
Bonvillain RW, Scarritt ME, Pashos NC, Mayeux JP, Meshberger CL, Betancourt AM, et al. Non human primate lung decellularization and recellularization using a specialized large-organ bioreactor. J Vis Exp. 2012;18:2437–52.
Yang B, Zhang Y, Zhou L, Sun Z, Zheng J, Chen Y, et al. Development of a porcine bladder acellular matrix with well-preserved extracellular bioactive factors for tissue engineering. Tissue Eng Part C Methods. 2010;16:1201–11.
Kaiser NJ, Coulombe KLK. Physiologically inspired cardiac scaffolds for tailored in vivo function and heart regeneration. Biomed Mater. 2015;10:1–26.
Abd-Elgaliel WR, Tung CH. A cardiac tissue-specific binding agent of troponin I. Mol Biosyst. 2012;8:2629–32.
Wiles K, Fishman JM, De Coppi P, Birchall MA. The host immune response to tissue-engineered organs: Current problems and future directions. Tissue Eng Part B Rev. 2016;22:208–19.
Wang Y, Bao J, Wu X, Wu Q, Li Y, Zhou Y, et al. Genipin crosslinking reduced the immunogenicity of xenogeneic decellularized porcine whole-liver matrices through regulation of immune cell proliferation and polarization. Sci Rep. 2016;6:1–16.
Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2009;20:109–16.
Acknowledgements
Work on this paper was financially supported by Ministry of Science, Industry and Technology, Republic of Turkey (Project ID. 0089.TGSD.2013) and the Scientific and Technological Research Council of Turkey (Project ID. 114S851). We wish to thank Canakkale Onsekiz Mart University, Science and Technology Application & Research Center and MER-TER Medical for collaborating with analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Arslan, Y.E., Galata, Y.F., Sezgin Arslan, T. et al. Trans-differentiation of human adipose-derived mesenchymal stem cells into cardiomyocyte-like cells on decellularized bovine myocardial extracellular matrix-based films. J Mater Sci: Mater Med 29, 127 (2018). https://doi.org/10.1007/s10856-018-6135-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-018-6135-4