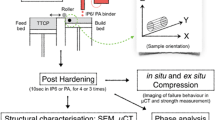
Abstract
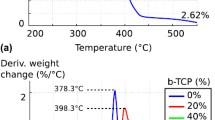
The development of 3D printing hardware, software and materials has enabled the production of bone substitute scaffolds for tissue engineering. Calcium phosphates cements, such as those based on α-tricalcium phosphate (α-TCP), have recognized properties of osteoinductivity, osteoconductivity and resorbability and can be used to 3D print scaffolds to support and induce tissue formation and be replaced by natural bone. At present, however, the mechanical properties found for 3D printed bone scaffolds are only satisfactory for non-load bearing applications. This study varied the post-processing conditions of the 3D powder printing process of α-TCP cement scaffolds by either immersing the parts into binder, Ringer’s solution or phosphoric acid, or by sintering in temperatures ranging from 800 to 1500 °C. The porosity, composition (phase changes), morphology, shrinkage and compressive strength were evaluated. The mechanical strength of the post-processed 3D printed scaffolds increased compared to the green parts and was in the range of the trabecular bone. Although the mechanical properties achieved are still low, the high porosity presented by the scaffolds can potentially result in greater bone ingrowth. The phases present in the scaffolds after the post-processing treatments were calcium-deficient hydroxyapatite, brushite, monetite, and unreacted α-TCP. Due to their chemical composition, the 3D printed scaffolds are expected to be resorbable, osteoinductive, and osteoconductive.
Graphical abstract










Similar content being viewed by others
References
Gerbino G, Biachi FA, Zavattero E, Tartara F, Garbossa D, Ducati A. Single-step resection and reconstruction using patient-specific implants in the treatment of benign cranio-orbital tumors. J Oral Maxillofac Surg. 2013; https://doi.org/10.1016/j.joms.2013.03.021.
Parthasarathy J. 3D modeling, custom implants and its future perspectives in craniofacial surgery. Annals Maxillofac Surg. 2014; https://doi.org/10.4103/2231-0746.133065.
Brunello G, Sivolella S, Meneghello R, Ferroni L, Gardin C, Piattelli A, Zavan B, Bressan E. Powder-based 3D printing for bone tissue engineering. Biotechnol Adv. 2016; https://doi.org/10.1016/j.biotechadv.2016.03.009.
Shao H, He Y, Fu J, He D, Yang X, Xie J, Yao C, Ye J, Xu S, Gou Z. 3D printing magnesium-doped wollastonite/ß-TCP bioceramics scaffolds with high strength and adjustable degradation. J Eur Ceram Soc. 2016; https://doi.org/10.1016/j.jeurceramsoc.2016.01.010.
Inzana JA, Olvera D, Fuller SM, Kelly JP, Graeve OA, Schwartz EM, Kates SL, Awad HA. 3D Printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. 2014; https://doi.org/10.1016/j.biomaterials.2014.01.064.
Butscher A, Bohner M, Hofmann S, Gaucler L, Müller R. Structural and material approaches to bone tissue engineering in powder-based three-dimensional printing. Acta Biomater. 2011; https://doi.org/10.1016/j.actbio.2010.09.039.
Butscher A, Bohner M, Doebelin N, Hofmann S, Müller R. New depowdering-friendly designs for three-dimensional printing of calcium phosphate bone substitutes. Acta Biomater. 2013; https://doi.org/10.1016/j.actbio.2013.07.019.
Montazerian H, Davoodi E, Asadi-Eydivand M, Kadkhodapour J, Solati-Hashjin M. Porous scaffold internal architecture design based on minimal surfaces: a compromise between permeability and elastic properties. Mater Des. 2017; https://doi.org/10.1016/j.matdes.2017.04.009.
Afshar M, Anaraki AP, Montazerian H, Kadkhodapour J. Additive manufacturing and mechanical characterization of graded porosity scaffolds designed based on triply periodic minimal surface architectures. J Mech Behav Biomed Mater. 2016; https://doi.org/10.1016/j.jmbbm.2016.05.027.
Munsch M. Laser additive manufacturing of customized prosthetics and implants for biomedical applications. In: Brandt M, editor. Laser Additive Manufacturing - Materials, Design, Technologies, and Applications, Woodhead Publishing Series in Electronic and Optical Materials. Cambridge, UK: Elsevier Science & Technology, 2017. p. 399–420.
Jardin LA, Larosa MA, Kaasi A, Kharmandayan P. Additive manufacturing in medicine. Ref Module Mater Sci Mater Eng. 2017; https://doi.org/10.1016/B978-0-12-803581-8.04152-7.
Jardini AL, Larosa MA, Maciel Filho R, Zavaglia AAC, Bernardes LF, Lambert CS, Calderoni DR, Kharmandayana P. Cranial reconstruction: 3D biomodel and custom-built implant created using additive manufacturing. J Cranio-Maxillofac Surg. 2014; https://doi.org/10.1016/j.jcms.2014.07.006.
Rachmiel A, Shilo D, Blanc O, Emodi O. Reconstruction of complex mandibular defects using integrated dental custom-made titanium implants. Br J Oral Maxillofac Surg. 2017; https://doi.org/10.1016/j.bjoms.2017.01.006.
Bertol LS, Kindlein Jr W, Silva FP, Aumund-Kopp K. Medical design: direct metal laser sintering of Ti-6Al-4V. Mater Des. 2010; https://doi.org/10.1016/j.matdes.2010.02.050.
Visscher DO, Farre-Guasch E, Helder MN, Gibbs S, Forouzanfar T, van Zuijlen PP, Wolff J. Advances in bioprinting technologies for craniofacial reconstruction. Trends Biotechnol. 2016; https://doi.org/10.1016/j.tibtech.2016.04.001.
Leong KF, Cheah CM, Chua CK. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement of tissues and organs. Biomaterials. 2003; https://doi.org/10.1016/S0142-9612(03)00030-9.
Lam CXF, Mo XM, Teoh SH, Hutmacher DW. Scaffold development using 3D printing with a starch-based polymer. Mater Sci Eng C. 2002; https://doi.org/10.1016/S0928-4931(02)00012-7.
Carrodeguas RG, De Aza S. α-Tricalcium phosphate: synthesis, properties and biomedical applications. Acta Biomater. 2011; https://doi.org/10.1016/j.actbio.2011.06.019.
Dorozhkin SV. Bioceramics of calcium orthophosphates. Biomaterials. 2010; https://doi.org/10.1016/j.biomaterials.2009.11.050.
Castilho M, Dias M, Vorndran E, Gbureck U, Fernandes P, Pires I, Gouveia B, Armés H, Pires E, Rodrigues J. Application of a 3D printed customized implant for canine cruciate ligament treatment by tibial tuberosity advancement. Biofabrication. 2014; https://doi.org/10.1088/1758-5082/6/2/025005.
Gbureck U, Hozel T, Klammert U, Wurzler K, Muller FA, Barralet JE. Resorbable dicalcium phosphate bone substitutes prepared by 3D powder printing. Adv Funct Mater. 2007; https://doi.org/10.1002/adfm.200700019.
Bose S, Vahabzadeh S, Bandyopadhyay A. Bone tissue engineering using 3D printing. Mater Today. 2013; https://doi.org/10.1016/j.mattod.2013.11.017.
Almela T, Brook IM, Khoshroo K, Rasoulianboroujeni M, Fahimipour F, Tahriri M, Dashtimoghadam E, El-Awa A, Tayebi L, Moharamzadeh K. Simulation of cortico-cancellous bone structure by 3D printing of bilayer calcium phosphate-based scaffolds. Bioprinting. 2017; https://doi.org/10.1016/j.bprint.2017.04.001.
Bertol LS, Schabbach R, Santos LAL. Dimensional evaluation of patient-specific 3D printing using calcium phosphate cement for craniofacial bone reconstruction. J Biomater Appl. 2017; https://doi.org/10.1177/0885328216682672.
Klammert U, Gbureck U, Vorndran E, Rödiger J, Meyer-Marcotty P, Kübler AC. 3D powder printed calcium phosphate implants for reconstruction of cranial and maxillofacial defects. J Cranio-Maxillofac Surg. 2010; https://doi.org/10.1016/j.jcms.2010.01.009.
Burguera EF, Xu HH, Takagi S, Chow LC. High early strength calcium phosphate bone cement: effects of dicalcium phosphate dihydrate and absorbable fibers. J Biomed Mater Res. 2005; https://doi.org/10.1002/jbm.a.30497.
Mauffrey C, Seligson D, Lichte P, Pape HC, Al-Rayyan M. Bone graft substitutes for articular supoprt and metaphyseal comminution: what are the options? Injury, Int J Care Injured. 2011; https://doi.org/10.1016/j.injury.2011.06.012.
Tamimi F, Torres J, Gbureck U, Lopez-Cabarcos E, Bassett DC, Alkhraisat MH, Barralet J. Craniofacial vertical bone augmentation: a comparison between 3D printed monolithic monetite blocks and autologous onlay grafts in the rabbit. Biomaterials. 2009; https://doi.org/10.1016/j.biomaterials.2009.07.049.
Bergmann C, Lindner M, Zhang W, Koczurischer H. 3D printing of bone substitute implants using calcium phosphate and bioactive glasses. J Eur Ceram Soc. 2010; https://doi.org/10.1016/j.jeurceramsoc.2010.04.037.
Staffa G, Barbanera A, Faiola A, Fricia M, Limoni P, Mottaran R, Zanotti B, Stefini R. Custom made bioceramic implants in complex and large cranial reconstruction: a two-year follow-up. J Cranio-Maxillofac Surg. 2012; https://doi.org/10.1016/j.jcms.2011.04.014.
Durucan C, Brown PW. Reactivity of alfa-tricalcium phosphate. J Mater Sci. 2002; https://doi.org/10.1023/A:1014347814241.
Ginebra MP, Fernandez E, De Maeyer EAP, Verbeeck RMH, Boltong MG, Ginebra J, Driessens FCM, Planell JA. Setting reaction and hardening of an apatitic calcium phosphate cement. J Dent Res. 1997; https://doi.org/10.1177/00220345970760041201.
Cama G. Calcium phosphate cements for bone regeneration. In: Dubruel P, Van Vlierberghe S, editors. Biomaterials for bone regeneration. Cambridge, UK: Woodhead Publishing; 2014. p. 3–25.
Tamimi F, Torres J, Lopez-Cabarcos E, Basset DC, Habibovic P, Luceron E, Barralet JE. Minimally invasive maxillofacial vertical bone augmentation using brushite based cements. Biomaterials. 2009, https://doi.org/10.1016/j.biomaterials.2008.09.032.
Uhland SA, Holman RK, Morissette S, Cima MJ, Sachs EM. Strength of green ceramics with low binder content. J Am Ceram Soc. 2001; https://doi.org/10.1111/j.1151-2916.2001.tb01098.x.
Cox CS, Thornby JA, Gibbons GJ, Williams MA, Mallick KK. 3D printing of porous hydroxyapatite scaffolds intended for use in bone tissue applications. Mater Sci Eng C. https://doi.org/10.1016/j.msec.2014.11.024.
Butscher A, Bohner M, Roth C, Ernstberger A, Heuberger R, Doebelin N, Rohr PR, Müller R. Printability of calcium phosphate powders for three-dimensional printing of tissue engineering scaffolds. Acta Biomater. 2012; https://doi.org/10.1016/j.actbio.2011.08.027.
Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005; https://doi.org/10.1016/j.biomaterials.2005.02.002.
Tarafder S, Balla VK, Davies NM, Bandyopadhyay A, Bose S. Microwave-sintered 3D printed tricalcium phosphate scaffolds for bone tissue engineering. J Tissue Eng Regen Med. 2012; https://doi.org/10.1002/term.555.
Will J, Melcher R, Treul C, Travitzky N, Kneser U, Polykandriotis E, Horch R, Greil P. Porous ceramic bone scaffolds for vascularized bone tissue regeneration. J Mater Sci Mater Med. 2008; https://doi.org/10.1007/s10856-007-3346-5.
Zhou Z, Buchanan F, Mitchell C, Dunne N. Printability of calcium phosphate: calcium sulphate powders for the application of tissue engineered bone scaffolds using the 3D printing technique. Mater Sci Eng C. 2014; https://doi.org/10.1016/j.msec.2014.01.027.
Leukers B, Gülkan H, Irsen SH, Milz S, Tille C, Schieker M, Seitz H. Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing. J Mater Sci Mater Med. 2005; https://doi.org/10.1007/s10856-005-4716-5.
Acknowledgements
The authors would like to thank the National Institute of Biofabrication (INCT-BIOFABRIS), Brazil. This work was supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil) and FINEP (Financiadora de Estudos e Projetos, Brazil).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Bertol, L.S., Schabbach, R. & Loureiro dos Santos, L.A. Different post-processing conditions for 3D bioprinted α-tricalcium phosphate scaffolds. J Mater Sci: Mater Med 28, 168 (2017). https://doi.org/10.1007/s10856-017-5989-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-017-5989-1