Abstract
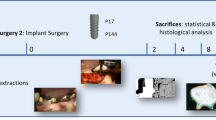
This study aimed to evaluate the in vivo osseointegration of implants with hydrophobic antimicrobial GL13K-peptide coating in rabbit femoral condyles by micro-CT and histological analysis. Six male Japanese Rabbits (4 months old and weighing 2.5 kg each) were included in this study. Twelve implants (3.75 mm wide, 7 mm long) were randomly distributed in two groups, with six implants in the experimental group coated with GL13K peptide and six implants in the control group without surface coating. Each implant in the test and the control group was randomly implanted in the left or right side of femoral condyles. On one side randomly-selected of the femur, each rabbit received a drill that was left without implant as control for the natural healing of bone. After 3 weeks of healing radiographic evaluation of the implant sites was taken. After 6 weeks of healing, rabbits were sacrificed for evaluation of the short-term osseointegration of the dental implants using digital radiography, micro-CT and histology analysis. To perform evaluation of osseointegration, implant location and group was double blinded for surgeon and histology/radiology researcher. Two rabbits died of wound infection in sites with non-coated implants 2 weeks after surgery. Thus, at least four rabbits per group survived after 6 weeks of healing. The wounds healed without suppuration and inflammation. No implant was loose after 6 weeks of healing. Radiography observations showed good osseointegration after 3 and 6 weeks postoperatively, which proved that the tissues followed a natural healing process. Micro-CT reconstruction and analysis showed that there was no statistically significant difference (P > 0.05) in volume of bone around the implant between implants coated with GL13K peptide and implants without coating. Histomorphometric analysis also showed that the mineralized bone area was no statistically different (P > 0.05) between implants coated with GL13K peptide and implants without coating. This study demonstrates that titanium dental implants with an antimicrobial GL13K coating enables in vivo implant osseointegration at similar bone growth rates than gold-standard non-coated dental implants up to 6 weeks of implantation in rabbit femurs.






Similar content being viewed by others
References
Dabdoub SM, Tsigarida AA, Kumar PS. Patient-specific analysis of periodontal and peri-implant microbiomes. J Dent Res. 2013;92(S12):168s–75s. doi:10.1177/0022034513504950.
Group MR. Annual industry report. Implant Dent. 2000;9:192–4.
Lindquist LW, Carlsson GE, Jemt T. A prospective 15-year follow-up study of mandibular fixed prostheses supported by osseointegrated implants—clinical results and marginal bone loss. Clin Oral Implant Res. 1996;7(4):329–36. doi:10.1034/j.1600-0501.1996.070405.x.
Schwartz-Arad D, Kidron N, Dolev E. A long-term study of implants supporting overdentures as a model for implant success. J Periodontol. 2005;76(9):1431–5. doi:10.1902/jop.2005.76.9.1431.
Lindquist LW, Carlsson GE, Jemt T. A prospective 15-year follow-up study of mandibular fixed prostheses supported by osseointegrated implants. Clinical results and marginal bone loss. Clin Oral Implants Res. 1996;7(4):329–36.
Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol. 2008;35:286–91. doi:10.1111/j.1600-051X.2008.01274.x.
Wu Y, Zitelli JP, TenHuisen KS, Yu XJ, Libera MR. Differential response of Staphylococci and osteoblasts to varying titanium surface roughness. Biomaterials. 2011;32(4):951–60. doi:10.1016/j.biomaterials.2010.10.001.
Cheng SJ, Tseng IY, Lee JJ, Kok SH. A prospective study of the risk factors associated with failure of mini-implants used for orthodontic anchorage. Int J Oral Max Impl. 2004;19(1):100–6.
Isidor F. Histological evaluation of peri-implant bone at implants subjected to occlusal overload or plaque accumulation. Clin Oral Implant Res. 1997;8(1):1–9. doi:10.1111/j.1600-0501.1997.tb00001.x.
Branemark PI, Hansson BO, Adell R, Breine U, Lindstrom J, Hallen O, et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl. 1977;16:1–132.
Arciniegas M, Aparicio C, Manero JM, Gil FJ. Low elastic modulus metals for joint prosthesis: Tantalum and nickel-titanium foams. J Eur Ceram Soc. 2007;27(11):3391–8. doi:10.1016/j.jeurceramsoc.2007.02.184.
Bumgardner JD, Adatrow P, Haggard WO, Norowski PA. Emerging antibacterial biomaterial strategies for the prevention of peri-implant inflammatory diseases. Int J Oral Maxillofac Implants. 2011;26(3):553–60.
Le Guehennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007;23(7):844–54. doi:10.1016/j.dental.2006.06.025.
Schliephake H, Scharnweber D. Chemical and biological functionalization of titanium for dental implants. J Mater Chem. 2008;18(21):2404–14. doi:10.1039/015355b.
Chen X, Li YP, Aparicio C: Biofunctional coatings for dental implants. In: Nazarpour S, Chaker M, editors. Thin films and coatings in biology. Biological and medical physics, biomedical engineering series. New York: Springer; 2013. p. 105–43.
Jahoda D, Nyc O, Pokorny D, Landor I, Sosna A. Antibiotic treatment for prevention of infectious complications in joint replacement. Acta Chir Orthop Traumatol Cech. 2006;73(2):108–14.
Kim WH, Lee SB, Oh KT, Moon SK, Kim KM, Kim KN. The release behavior of CHX from polymer-coated titanium surfaces. Surf Interface Anal. 2008;40(3-4):202–4. doi:10.1002/Sia.2809.
Chen W, Liu Y, Courtney HS, Bettenga M, Agrawal CM, Bumgardner JD, et al. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials. 2006;27(32):5512–7. doi:10.1016/j.biomaterials.2006.07.003.
Harris LG, Tosatti S, Wieland M, Textor M, Richards RG. Staphylococcus aureus adhesion to titanium oxide surfaces coated with non-functionalized and peptide-functionalized poly(l-lysine)-grafted-poly(ethylene glycol) copolymers. Biomaterials. 2004;25(18):4135–48. doi:10.1016/j.biomaterials.2003.11.033.
Chua PH, Neoh KG, Kang ET, Wang W. Surface functionalization of titanium with hyaluronic acid/chitosan polyelectrolyte multilayers and RGD for promoting osteoblast functions and inhibiting bacterial adhesion. Biomaterials. 2008;29(10):1412–21. doi:10.1016/j.biomaterials.2007.12.019.
Neu HC. The crisis in antibiotic-resistance. Science. 1992;257(5073):1064–73. doi:10.1126/science.257.5073.1064.
Abdolhosseini M, Nandula SR, Song J, Hirt H, Gorr SU. Lysine substitutions convert a bacterial-agglutinating peptide into a bactericidal peptide that retains anti-lipopolysaccharide activity and low hemolytic activity. Peptides. 2012;35(2):231–8. doi:10.1016/j.peptides.2012.03.017.
Hirt H, Gorr SU. Antimicrobial peptide GL13K is effective in reducing biofilms of pseudomonas aeruginosa. Antimicrob Agents Chemother. 2013;57(10):4903–10. doi:10.1128/Aac.00311-13.
Holmberg KV, Abdolhosseini M, Li Y, Chen X, Gorr SU, Aparicio C. Bio-inspired stable antimicrobial peptide coatings for dental applications. Acta Biomater. 2013. 10.1016/j.actbio.2013.06.017.
Lamont RJ, Jenkinson HF. Life below the gum line: pathogenic mechanisms of porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62(4):1244–63.
Chen X, Hirt H, Li YP, Gorr SU, Aparicio C. Antimicrobial GL13K peptide coatings killed and ruptured the wall of streptococcus gordonii and prevented formation and growth of biofilms. PLos One. 2014. doi:ARTN e11157910.1371/journal.pone.0111579.
Cook GS, Costerton JW, Lamont RJ. Biofilm formation by Porphyromonas gingivalis and Streptococcus gordonii. J Periodontal Res. 1998;33(6):323–7. doi:10.1111/j.1600-0765.1998.tb02206.x.
Chen X, Sevilla P, Aparicio C. Surface biofunctionalization by covalent co-immobilization of oligopeptides. Colloid Surf B. 2013;107:189–97. doi:10.1016/j.colsurfb.2013.02.005.
Chen X. Multi-bioactive peptide coatings for dental implants. University of Minnesota Digital Conservancy; 2014. http://hdl.handle.net/11299/173938. Accessed 28 Dec 2016.
Gorr SU, Abdolhosseini M. Antimicrobial peptides and periodontal disease. J Clin Periodontol. 2011;38:126–41. doi:10.1111/j.1600-051X.2010.01664.x.
Krisanaprakornkit S, Khongkhunthian S. The Role of Antimicrobial Peptides in Periodontal Disease. In: Walchuck R, editor. Periodontitis: Symptoms, Treatment and Prevention. Public Health in the 21st Century; 2010, p. 73–103.
Hancock REW, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24(12):1551–7. doi:10.1038/Nbt1267.
Svensson S, Suska F, Emanuelsson L, Palmquist A, Norlindh B, Trobos M, et al. Osseointegration of titanium with an antimicrobial nanostructured noble metal coating. Nanomedicine. 2013;9(7):1048–56. doi:10.1016/j.nano.2013.04.009.
Moojen DJF, Vogely HC, Fleer A, Nikkels PGJ, Higham PA, Verbout AJ, et al. Prophylaxis of infection and effects on osseointegration using a tobramycin-periapatite coating on titanium implants—an experimental study in the rabbit. J Orthop Res. 2009;27(6):710–6. doi:10.1002/jor.20808.
Wang LX, He S, Wu XM, Liang SS, Mu ZL, Wei J, et al. Polyetheretherketone/nano-fluorohydroxyapatite composite with antimicrobial activity and osseointegration properties. Biomaterials. 2014;35(25):6758–75. doi:10.1016/j.biomaterials.2014.04.085.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest.
Rights and permissions
About this article
Cite this article
Chen, X., Zhou, X., Liu, S. et al. In vivo osseointegration of dental implants with an antimicrobial peptide coating. J Mater Sci: Mater Med 28, 76 (2017). https://doi.org/10.1007/s10856-017-5885-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-017-5885-8