Abstract
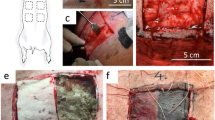
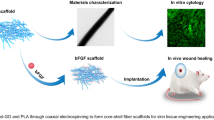
In recent years, temporary skin grafts (TSG) based on natural biopolymers modified with carbon nanostructures have received considerable attention for wound healing. Developments are required to improve physico-mechanical properties of these materials to match to natural skins. Additionally, in-deep pre-clinical examinations are necessary to ensure biological performance and toxicity effect in vivo. In the present work, we show superior acute-wound healing effect of graphene oxide nanosheets embedded in ultrafine biopolymer fibers (60 nm) on adult male rats. Nano-fibrous chitosan-based skin grafts crosslinked by Genepin with physico-mechanical properties close to natural skins were prepared by electrospinning of highly concentrated chitosan- polyvinylpyrrolidone solutions containing graphene oxide (GO) nanosheets. No surfactants and organic solvents were utilized to ensure high biocompatibility of the fibrous structure. In vitro evaluations by human skin fibroblast cells including live and dead assay and MTT results show that GO promote cell viability of porous nanofibrous membrane while providing enhanced bactericidal capacity. In vivo studies on rat’s skin determine accelerated healing effect, i.e. a large open wound (1.5 × 1.5 cm2) is fully regenerated after 14-day of post operation while healing is observed for sterile gauze sponge (as the control). Pathological studies support thick dermis formation and complete epithelialization in the presence of 1.5 wt% GO nanosheets. Over 99% wound healing occurs after 21 days for the injury covered with TSG containing 1.5 wt% GO while this would takes weeks for the control. Therefore, the developed materials have a high potential to be used as TSG as pre-clinical testing has shown.
Graphical Abstract








Similar content being viewed by others
References
Venugopal J, Ramakrishna S. Applications of polymer nanofibers in biomedicine and biotechnology. Appl Biochem Biotechnol. 2005;125(3):147–57.
Vasconcelos A, Cavaco-Paulo A. Wound dressings for a proteolytic-rich environment. Appl Microbiol Biotechnol. 2011;90(2):445–60. doi:10.1007/s00253-011-3135-4
Tamayol A, Akbari M, Zilberman Y, Comotto M, Lesha E, Serex L, et al. Flexible pH‐sensing hydrogel fibers for epidermal applications. Adv Healthcare Mater. 2016;5(6):711–9.
Mogoşanu GD, Grumezescu AM. Natural and synthetic polymers for wounds and burns dressing. Int J Pharm. 2014;463(2):127–36.
Tamayol A, Akbari M, Annabi N, Paul A, Khademhosseini A, Juncker D. Fiber-based tissue engineering: progress, challenges, and opportunities. Biotechnol Adv. 2013;31(5):669–87.
Mahmoudi N, Ostadhossein F, Simchi A. Physicochemical and antibacterial properties of chitosan‐polyvinylpyrrolidone films containing self‐organized graphene oxide nanolayers. J Appl Polym Sci 2016;133(11):43194.
Kim I-Y, Seo S-J, Moon H-S, Yoo M-K, Park I-Y, Kim B-C, et al. Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv. 2008;26(1):1–21.
Jafari J, Emami SH, Samadikuchaksaraei A, Bahar MA, Gorjipour F. Electrospun chitosan-gelatin nanofiberous scaffold: fabrication and in vitro evaluation. Biomed Mater Eng. 2011;21(2):99–112. doi:10.3233/bme-2011-0660
Azad AK, Sermsintham N, Chandrkrachang S, Stevens WF. Chitosan membrane as a wound‐healing dressing: characterization and clinical application. J Biomed Mater Res Part B Appl Biomater. 2004;69(2):216–22.
Jayakumar R, Prabaharan M, Nair S, Tamura H. Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol Adv. 2010;28(1):142–50.
Goenka S, Sant V, Sant S. Graphene-based nanomaterials for drug delivery and tissue engineering. J Control Release. 2014;173:75–88.
Yao J, Sun Y, Yang M, Duan Y. Chemistry, physics and biology of graphene-based nanomaterials: new horizons for sensing, imaging and medicine. J Mater Chem. 2012;22(29):14313–29.
Chung C, Kim Y-K, Shin D, Ryoo S-R, Hong BH, Min D-H. Biomedical applications of graphene and graphene oxide. Acc Chem Res. 2013;46(10):2211–24.
Terzopoulou Z, Kyzas GZ, Bikiaris DN. Recent advances in nanocomposite materials of graphene derivatives with polysaccharides. Mater. 2015;8(2):652–83.
Ionita M, Crica L, Tiainen H, Haugen H, Vasile E, Dinescu S, et al. Gelatin–poly (vinyl alcohol) porous biocomposites reinforced with graphene oxide as biomaterials. J Mater Chem B. 2016;4(2):282–91.
Murray E, Sayyar S, Thompson BC, Gorkin R III, Officer DL, Wallace GG. A bio-friendly, green route to processable, biocompatible graphene/polymer composites. RSC Adv. 2015;5(56):45284–90.
Ye Y-S, Zeng H-X, Wu J, Dong L-Y, Zhu J-T, Xue Z-Get al. Biocompatible reduced graphene oxide sheets withsuperior water dispersibility stabilized by cellulose nanocrystals and their polyethylene oxide composites. Green Chemistry. 2016;18:1674–83.
Wang C, Li Y, Ding G, Xie X, Jiang M. Preparation and characterization of graphene oxide/poly(vinyl alcohol) composite nanofibers via electrospinning. J Appl Polym Sci. 2013;127(4):3026–32. doi:10.1002/app.37656.
Jin L, Wu D, Kuddannaya S, Zhang Y, Wang Z. Fabrication, characterization, and biocompatibility of polymer cored reduced graphene oxide nanofibers. ACS Appl Mater Interfaces. 2016;8(8):5170–7.
Liu Y, Park M, Shin HK, Pant B, Choi J, Park YW, et al. Facile preparation and characterization of poly(vinyl alcohol)/chitosan/graphene oxide biocomposite nanofibers. J Ind Eng Chem. 2014;20(6):4415–20. doi:10.1016/j.jiec.2014.02.009.
Lu B, Li T, Zhao H, Li X, Gao C, Zhang S, et al. Graphene-based composite materials beneficial to wound healing. Nanoscale. 2012;4(9):2978–82. doi:10.1039/c2nr11958g.
Azarniya A, Eslahi N, Mahmoudi N, Simchi A. Effect of graphene oxide nanosheets on the physico-mechanical properties of chitosan/bacterial cellulose nanofibrous composites. Compos Part A. 2016;85:113–22.
de Faria AF, Perreault F, Shaulsky E, Arias Chavez LH, Elimelech M. Antimicrobial electrospun biopolymer nanofiber mats functionalized with graphene oxide–silver nanocomposites. ACS Appl Mater Interfaces. 2015;7(23):12751–9.
Mazaheri M, Akhavan O, Simchi A. Flexible bactericidal graphene oxide–chitosan layers for stem cell proliferation. Appl Surf Sci. 2014;301(0):456–62. doi:10.1016/j.apsusc.2014.02.099.
Mahmoudi N, Simchi A. On the biological performance of graphene oxide-modified chitosan/polyvinyl pyrrolidone nanocomposite membranes: in vitro and in vivo effects of graphene oxide. Mater Sci Eng. 2017;70:121–31.
Hummers WS Jr, Offeman RE. Preparation of graphitic oxide. J Am Chem Soc. 1958;80(6):1339
Mi FL, Sung HW, Shyu SS. Synthesis and characterization of a novel chitosan‐based network prepared using naturally occurring crosslinker. J Polym Sci Part A. 2000;38(15):2804–14.
Vega-Avila E, Pugsley MK, editors. An overview of colorimetric assay methods used to assess survival or proliferation of mammalian cells. Proc West Pharmacol Soc. 2011.
Care IoLARCo, Animals UoL, resources NIoHDoR. Guide forthe care and use of laboratory animals. National Academies.1985;1:89–544.
Gilchrist MD, Ní Annaidh A, Destrade M. Characterising the anisotropic mechanical properties of excised human skin. J Mech Behav Biomed Mater. 2012;5(1):139–48.
Erencia M, Cano F, Tornero JA, Fernandes MM, Tzanov T, Macanás J, et al. Electrospinning of gelatin fibers using solutions with low acetic acid concentration: effect of solvent composition on both diameter of electrospun fibers and cytotoxicity. J Appl Polym Sci 2015;132(25):42115.
Talukdar Y, Rashkow JT, Lalwani G, Kanakia S, Sitharaman B. The effects of graphene nanostructures on mesenchymal stem cells. Biomaterials. 2014;35(18):4863–77. doi:10.1016/j.biomaterials.2014.02.054
Kim J, Kim Y-R, Kim Y, Lim KT, Seonwoo H, Park S, et al. Graphene-incorporated chitosan substrata for adhesion and differentiation of human mesenchymal stem cells. J Mater Chem B. 2013;1(7):933–8. doi:10.1039/C2TB00274D
Poursamar SA, Lehner AN, Azami M, Ebrahimi-Barough S, Samadikuchaksaraei A, Antunes AP. The effects of crosslinkers on physical, mechanical, and cytotoxic properties of gelatin sponge prepared via in-situ gas foaming method as a tissue engineering scaffold. Mater Sci Eng C Mater Biol Appl. 2016;63:1–9. doi:10.1016/j.msec.2016.02.034
Dalfino L, Giglio MT, Puntillo F, Marucci M, Brienza N. Haemodynamic goal-directed therapy and postoperative infections: earlier is better. A systematic review and meta-analysis. Crit Care. 2011;15(3):R154
Azuma K, Izumi R, Osaki T, Ifuku S, Morimoto M, Saimoto H, et al. Chitin, chitosan, and its derivatives for wound healing: old and new materials. J Funct Biomater. 2015;6(1):104–42.
Okamoto Y, Miyatake K, Morimoto M, Saimoto H, Shigemasa Y, Minami S. Mechanism of wound healing acceleration by chitin and chitosan. Res Adv Macromol. 2002;3:1–22.
Duan B, Yuan X, Zhu Y, Zhang Y, Li X, Zhang Y, et al. A nanofibrous composite membrane of PLGA–chitosan/PVA prepared by electrospinning. Eur Polym J. 2006;42(9):2013–22.
Ruiz ON, Fernando KS, Wang B, Brown NA, Luo PG, McNamara ND, et al. Graphene oxide: a nonspecific enhancer of cellular growth. ACS Nano. 2011;5(10):8100–7.
Acknowledgements
The authors acknowledge Dr. Fatemeh Mohandes, and Mr. Eng. Amin Jalali (Sharif university of Technology) for experimental and technical support. AS thanks funding support from Sharif University of Technology (No. G930305), Iran National Science Foundation (INSF No. 95-S-48740), and Iran Elite Foundation (No. ENL 5418).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Mahmoudi, N., Eslahi, N., Mehdipour, A. et al. Temporary skin grafts based on hybrid graphene oxide-natural biopolymer nanofibers as effective wound healing substitutes: pre-clinical and pathological studies in animal models. J Mater Sci: Mater Med 28, 73 (2017). https://doi.org/10.1007/s10856-017-5874-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-017-5874-y