Abstract
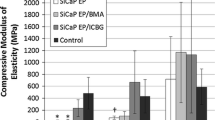
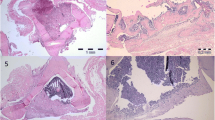
Bone substitutes have been a critical issue as the natural source can seldom provide enough bone to support full healing. No bone substitute complies with all necessary functions and characteristics that an autograft does. Polyurethane sponges have been used as a surgical alternative to cancellous bone grafts for critical bone defect donor sites. Critical bone defects were created on the tibial tuberosity and iliac crest using an ovine model. In group I (control-untreated), no bone regeneration was observed in any animal. In group II (defects left empty but covered with a microporous polymeric membrane), the new bone bridged the top ends in all animals. In groups III and IV, bone defects were implanted with polyurethane scaffolds modified with biologically active compounds, and bone regeneration was more efficient than in group II. In groups III and IV there were higher values of bone regeneration specific parameters used for evaluation (P < 0.05) although the comparison between these groups was not possible. The results obtained in this study suggest that biodegradable polyurethane substitutes modified with biologically active substances may offer an alternative to bone graft, reducing donor site morbidity associated with autogenous cancellous bone harvesting.
Graphical Abstract















Similar content being viewed by others
References
Faour O, Dimitriou R, Cousins CA, Giannoudis PV. The use of bone graft substitutes in large cancellous voids: any specific needs? Injury. 2011;42(S2):S87–90.
Blockhuis TJ, Chris Arts JJ. Bioactive and osteoinductive bone graft substitutes: definitions, facts and myths. Injury. 2011;42(S4):S26–9.
Saito N, Takaoka K. New synthetic biodegradable polymers as BMP carriers for bone tissue engineering. Biomaterials. 2003;24(13):2287–93.
Gogolewski S, Gorna K. Biodegradable polyurethane cancellous bone graft substitutes in the treatment of iliac crest defects. J Biomed Mater Res, Part A. 2007;80(1):94–101.
Kroeze RJ, Helder MN, Govaert LE, Smit TH. Biodegradable polymers in bone tissue engineering. Materials. 2009;2(3):833–56.
Sabir MI, Xu X, Li L. A review on biodegradable polymeric materials for bone tissue engineering applications. J Mater Sci. 2009;44(21):5713–24.
Razak SIA, Fadzliana N, Sharif A, Aizan W, Rahman WAWA. Biodegradable polymers and their bone applications: a review. Int J Basic Appl Sci. 2012;12(1):31–49.
Sheikh Z, Najeeb S, Khurshid Z, Verma V, Rashid H, Glogauer M. Biodegradable materials for bone repair and tissue engineering applications. Materials. 2015;8(9):5744–94.
Hannink G, Arts CJJ. Bioresorbability, porosity and mechanical strength of bone substitutes: what is optimal for bone regeneration? Injury. 2011;42(4):22–5.
Gogolewski S, Gorna K. Biodegradable polyurethane cancellous bone graft substitutes in the treatment of iliac crest defects. J Biomed Mater Res A. 2007;80(1):94–101.
Sartori S, Chiono V, Tonda-Turo C, Mattu C, Ciardelli G. Biomimetic polyurethanes in nano and regenerative medicine. J Mater Chem B. 2014;2:5128–44.
Spaans CJ, Belgraver VW, Rienstra O, de Groot JH, Veth RP, Pennings AJ. Solvent-free fabrication of micro-porous polyurethane amide and polyurethane-urea scaffolds for repair and replacement of the knee-joint meniscus. Biomaterials. 2000;21(23):2453–60.
Gisselfält K, Edberg B, Flodin P. Synthesis and properties of degradable poly(urethaneurea)s to be used for ligament reconstructions. Biomacromolecules. 2002;3(5):951–8.
Kavlock KD, Pechar TW, Hollinger JO, Guelcher SA, Goldstein AS. Synthesis and characterization of segmented poly(esterurethane urea) elastomers for bone tissue engineering. Acta Biomater. 2007;3(4):475–84.
Thomas V, Kumari TV, Jayabalan M. In vitro studies on the effect of physical cross-linking on the biological performance of aliphatic poly (urethane urea) for blood contact applications. Biomacromolecules. 2001;2(2):588–96.
Gogolewski S; Rahn B; Wieling R. Bone regeneration in critical-size segmental diaphyseal defects implanted with bioresorbable polylactide bone substitute. Transactions. In: Proceedings of 27th Society for Biomaterials 27th Annual Meeting, Saint Paul, MN, USA, 2001; 24: p 572.
Gogolewski S, Gorna K, Turner S. Regeneration of bicortical defects in iliac crest of estrogen-deficient sheep, using new biodegradable polyurethane bone graft substitutes. J Biomed Mater earch Part A. 2006;77(4):802–10.
Gorna K, Gogolewski S. Biodegradable porous polyurethane scaffolds for tissue repair and regeneration. J Biomed Mater Res, Part A. 2006;79(1):128–38.
Gogolewski S, Gorna K, Zaczynska E, Czarny A. Structure-property relations and cytotoxicity of isosorbide-based biodegradable polyurethane scaffolds for tissue repair and regeneration. J Biomed Mater Res, Part A. 2008;85(2):456–65.
Lee CR, Grad S, Gorna K, Gogolewski K, Goessl A, Alini M. Fibrin-polyurethane composites for articular cartilage tissue engineering: a preliminary analysis. Tissue Eng. 2005;11:1562–73.
Guelcher S. Biodegradable polyurethanes: synthesis and applications in regenerative medicine. Tissue Eng Part B. 2008;14(1):3–17.
Schreader K, Bayer I, Milner D, Loth E, Jasiuk I. A polyurethane-based nanocomposite biocompatible bone adhesive. J Appl Polym Sci. 2013;127(6):4974–82.
Cherng JY, Hou TY, Shih MF, Talsma H, Hennink WE. Polyurethane-based drug delivery systems. Int J Pharm. 2013;450(1–2):145–62.
Woźniak P, Bil M, Ryszkowska K, Wychowański P, et al. Candidate bone-tissue-engineered product based on human-bone-derived cells and polyurethane scaffold. Acta Biomater. 2010;6(7):2484–93.
Potes JC, Reis JC, Silva FC, Relvas C, Cabrita AS, Simões JA. The Sheep as an animal model in orthopaedic research. Exp Pathol Health Sci. 2008;2(1):29–32.
Gorna K, Gogolewski S, Novel Biodegradable Polyurethanes for Medical Applications. In: Synthetic bioresorbable polymers for implants. ASTM STP 1396, Agrawal CM, Parr JE, Lin ST, Eds., ASTM, West Conshohocken, PA, 39–57, 2000.
Gorna K, Polowinski S, Gogolewski S. Synthesis and characterization of biodegradable poly(-caprolactone urethanes). I. The effect of the polyol molecular weight, catalyst and the chain extender on the molecular and physical characteristics. J Polym Sci Part A. 2002;40(1):156–70.
Gorna K, Gogolewski S. In vitro degradation of novel medical biodegradable aliphatic polyurethanes based on -caprolactone and Pluronics® with various hydrophilicities. Polym Degrad Stab. 2002;75(1):113–22.
Gorna K, Gogolewski S. Biodegradable polyurethanes for implants. II. In vitro degradation and calcification of materials from poly(-caprolactone) - poly(ethylene oxide) diols and various chain extenders. J Biomed Mater Res. 2002;60(4):592–606.
Gorna K, Gogolewski S. Preparation, degradation and calcification of biodegradable polyurethane foams for bone graft substitutes. J Biomed Mater Res, Part A. 2003;67A(3):813–27.
Schlickewei C, Verrier S, Lippross S, Pearce S, Alini M, Gogolewski S. Interaction of sheep bone marrow stromal cells with biodegradable polyurethane bone substitutes. Macromol Symp. 2007;253(1):162–71.
Gogolewski S, Biocompatible, biodegradable polyurethane materials with controlled hydrophobic to hydrophilic ratio, US Patent 8,460,378 B2.
Gugala Z, Gogolewski S. Regeneration of segmental diaphyseal defects in sheep tibiae using resorbable polymeric membranes: a preliminary study. J Orthop Trauma. 1999;13(3):187–95.
An YH, Bell TD. Experimental design, evaluation methods, data analysis and research ethics. In: An YH, Friedman RJ, editors. Animal models in orthopaedic research. Boca Raton: CRC Press; 1999. p. 15–37.
Pearce AI, Richards RG, Milz S, Schneider E, Pearce SG. Animal models for implant biomaterial research in bone: a review. Eur Cell Mater. 2007;13:1–10.
Schieker M, Seitz H, Drosse I, Seitz S, Mutschler W. Biomaterials as scaffold for bone tissue engineering. Eur J Trauma. 2006;32(2):114–24.
Rahn BA. Intra vital staining techniques. In: von Recum AF, editor. Handbook of biomaterials evaluation. 2nd ed. Philadelphia: Taylor & Francis; 1999. p. 727–42.
Coelho, PJV. Contribuição para o estudo da regeneração óssea mandibular consecutiva a perdas de substância – Tese de Doutoramento em Biomateriais. 2003. Faculdade de Medicina Dentária – Universidade de Lisboa. Lisboa. 268 pp.
Zedda M, Lepore G, Manca P, Chisu V, Farina V. Comparative Bone Histology of Adult Horses (Equus caballus) and Cows (Bos taurus). Anat Histol Embryol. 2008;37(6):442–5.
Tosta M, Cortes AR, Corrêa L, Pinto Ddos S Jr, Tumenas I, Katchburian E. Histologic and histomorphometric evaluation of a synthetic bone substitute for maxillary sinus grafting in humans. Oral Implants Res. 2013;24(8):866–70.
Bloebaum RD, Willie BM, Mitchell BS, Hofmann AA. Relationship between bone ingrowth, mineral apposition rate, and osteoblast activity. J Biomed Mater Res A. 2007;81(2):505–14.
Thorwarth M, Wehrhan F, Srour S, Schultze-Mosgau S, Felszeghy E, Bader RD, Schlegel KA. Evaluation of substitutes for bone: comparison of microradiographic and histologial assessments. Br J Oral Maxillofac Surg. 2007;45(1):41–7.
Gogolewski S, Pineda L, Büsing CM. Bone regeneration in segmental defects with resorbable polymeric membranes: IV. Does the polymer chemical composition affect the healing process? Biomaterials. 2000;21(24):2513–20.
Silbernagel JT, Kennedy SC, Johnson AL, Pijanowski GJ, Ehrhart N, Schaeffer D. Validation of canine cancellous and cortical polyurethane foam bone models. Vet and Comp Orthop Traumatol. 2002;15(4):200–4.
Jones A, Arns CH, Sheppard AP, Hutmacher DW, Milhorpe BK, Knackstedt MA. Assessment of bone ingrowth into porous biomaterials using MICRO-CT. Biomaterials. 2007;28(15):2491–504.
Rauch F, Travers R, Parfitt AM, Glorieux FH. Static and dynamic bone histomorphometry in children with osteogenesis imperfecta. Bone. 2000;26(6):581–9.
Gugala Z, Gogolewski S. In vitro growth and activity of primary chondrocytes on a resorbable polylactide three-dimensional scaffold. J Biomed Mater Res. 2000;49(2):183–91.
Gugala Z, Gogolewski S. Protein adsorption, attachment, growth and activity of primary rat osteoblasts on polylactide membranes with defined surface characteristics. Biomaterials. 2004;25(12):2341–51.
Gugala Z, Gogolewski S. Differentiation, growth and activity of rat bone marrow stromal cells on resorbable poly(L/DL-lactide) membranes. Biomaterials. 2004;25(12):2299–307.
Gugala Z, Gogolewski S. The in vitro growth and activity of sheep osteoblasts on three-dimensional scaffolds from poly(L/DL-lactide) 80/20%. J Biomed Mater Res, Part A. 2005;75(3):702–9.
Ip WY, Gogolewski S. Clinical application of resorbable polymers in guided bone regeneration. Macromol Symp. 2007;253(1):139–46.
Pineda LM, Büsing CM, Meinig RP, Gogolewski S. Bone regeneration with resorbable polymeric membranes. III. Effect of poly (L-lactide) membrane pore size on the bone healing process in large defects. J Biomed Mater Res. 1996;31:385–94.
Guedes e Silva CC, König B Jr, Carbonari MJ, Yoshimoto M, Allegrini S Jr, Bressiani JC. Tissue response around silicon nitride implants in rabbits. J Biomed Mater Res, Part A. 2008;84(2):337–43.
Hafeman AE, Li B, Yoshii T, Zienkiewicz K, Davidson JM, Guelcher SA. Injectable biodegradable polyurethane scaffolds with release of platelet-derived growth factor for tissue repair and regeneration. Pharm Res. 2008;25(10):2387–99.
McBane JE, Ebadi D, Sharifpoor S, Labow RS, Santerre JP. Differentiation of monocytes on a degradable, polar, hydrophobic, ionic polyurethane: two-dimensional films vs. three-dimensional scaffolds. Acta Biomater. 2011;7(1):115–22.
Acknowledgments
We would like to thank AO-ASIF for providing the needed consumables, namely India ink, calcein green and xylenol orange. We are grateful for the pertinent comments and fruitful discussion with all people involved. The participation of the Faculty of Medicine—University of Coimbra (Fernando Guerra), Oncology Portuguese Institute—IPO (Maria de Lurdes Orvalho), Faculty of Veterinary Medicine—University of Lisbon (António Ferreira e Sandra de Jesus) and Institute of Biomedical Technology—ITB (Isaura Geraldo) and Katarzyna Gorna is deeply acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors state that there is no conflict of interest of any nature.
Rights and permissions
About this article
Cite this article
Lavrador, C., Mascarenhas, R., Coelho, P. et al. Elastomeric enriched biodegradable polyurethane sponges for critical bone defects: a successful case study reducing donor site morbidity. J Mater Sci: Mater Med 27, 61 (2016). https://doi.org/10.1007/s10856-016-5667-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-016-5667-8