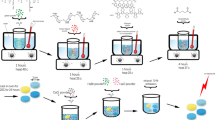
Abstract
The high degree of clinical routine in percutaneous transluminal coronary angioplasty (PTCA) with and without stenting has not changed the fact that a large number of coronary heart disease patients are still affected by post-operative complications such as restenosis and thrombosis. Because re-endothelialization is the crucial aspect of wound healing after cardiovascular implant surgery, there is a need for modern biomaterials to aid endothelial cells in their adhesion and functional recovery post-stenting. This study systematically examines the potential of numerous chemical polymer modifications with regard to endothelialization. Poly(ε-caprolactone) (PCL) and its chemically activated forms are investigated in detail, as well as the impact of polymer surface morphology and precoating with matrix protein. Human umbilical vein endothelial cells (HUVECs) are used to characterize endothelial cell responses in terms of in vitro viability and adhesion. As a potential component in drug eluting implants, VEGF is applied as stimulus to boost endothelial cell proliferation on the polymer. In conclusion, plasma chemical activation of PCL combined with VEGF stimulation best enhances in vitro endothelialization. Examining the impact of morphological, chemical and biological modifications of PCL, this study makes an important new contribution towards the existing body of work on polymer endothelialization.










Similar content being viewed by others
References
Dudash LA, Kligman F, Sarett SM, Kottke-Marchant K, Marchant RE. Endothelial cell attachment and shear response on biomimetic polymer-coated vascular grafts. J Biomed Mater Res. 2012;100(8):2204–10.
Waterhouse A, Wise SG, Yin Y, Wu B, James B, Zreiqat H, et al. In vivo biocompatibility of a plasma-activated, coronary stent coating. Biomaterials. 2012;33(32):7984–92.
Wu KK, Thiagarajan P. Role of endothelium in thrombosis and hemostasis. Annu Rev Med. 1996;47:315–31.
He Q, Zhao Y, Chen B, Xiao Z, Zhang J, Chen L, et al. Improved cellularization and angiogenesis using collagen scaffolds chemically conjugated with vascular endothelial growth factor. Acta Biomater. 2011;7(3):1084–93.
Jukema JW, Verschuren JJW, Ahmed TAN, Quax PHA. Restenosis after PCI. Part 1: pathophysiology and risk factors. Nat Rev Cardiol. 2011;9(1):53–62.
Sternberg K, Grabow N, Petersen S, Weitschies W, Harder C, Ince H, et al. Advances in coronary stent technology-active drug-loaded stent surfaces for prevention of restenosis and improvement of biocompatibility. Curr Pharm Biotechnol. 2013;14(1):76–90.
Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010;56(23):1897–907.
Wykrzykowska JJ, Onuma Y, Serruys PW. Advances in stent drug delivery: the future is in bioabsorbable stents. Expert Opin Drug Deliv. 2009;6(2):113–26.
Pitt CG, Gratzl MM, Kimmel GL, Surles J, Schindler A. Aliphatic polyesters II. The degradation of poly (DL-lactide), poly (epsilon-caprolactone), and their copolymers in vivo. Biomaterials. 1981;2(4):215–20.
Sun H, Mei L, Song C, Cui X, Wang P. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials. 2006;27(9):1735–40.
Berneel E, Desmet T, Declercq H, Dubruel P, Cornelissen M. Double protein-coated poly-ε-caprolactone scaffolds: successful 2D to 3D transfer. J Biomed Mater Res. 2012;100(7):1783–91.
Pektok E, Nottelet B, Tille J, Gurny R, Kalangos A, Moeller M, et al. Degradation and healing characteristics of small-diameter poly(ε-caprolactone) vascular grafts in the rat systemic arterial circulation. Circulation. 2008;118(24):2563–70.
Wulf K, Teske M, Löbler M, Luderer F, Schmitz K, Sternberg K. Surface functionalization of poly(ε-caprolactone) improves its biocompatibility as scaffold material for bioartificial vessel prostheses. J Biomed Mater Res. 2011;98(1):89–100.
Jiao Y, Cui F. Surface modification of polyester biomaterials for tissue engineering. Biomed Mater. 2007;2(4):24–7.
Goddard J, Hotchkiss J. Polymer surface modification for the attachment of bioactive compounds. Prog Polym Sci. 2007;32(7):698–725.
Isenberg BC, Williams C, Tranquillo RT. Engineering of small-diameter vessels. In: Atala A, Lanza R, Thomson JA, Nerem R, editors. Principles of regenerative medicine. 2nd ed. New York: Academic Press; 2011. p. 853–75.
de Mel A, Jell G, Stevens MM, Seifalian AM. Biofunctionalization of biomaterials for accelerated in situ endothelialization: a review. Biomacromolecules. 2008;9(11):2969–79.
Lin Q, Ding X, Qiu F, Song X, Fu G, Ji J. In situ endothelialization of intravascular stents coated with an anti-CD34 antibody functionalized heparin–collagen multilayer. Biomaterials. 2010;31(14):4017–25.
Cooper T, Sefton M. Fibronectin coating of collagen modules increases in vivo HUVEC survival and vessel formation in SCID mice. Acta Biomater. 2011;7(3):1072–83.
Adiguzel E, Ahmad PJ, Franco C, Bendeck MP. Collagens in the progression and complications of atherosclerosis. Vasc Med. 2009;14(1):73–89.
Çelebi B, Mantovani D, Pineault N. Effects of extracellular matrix proteins on the growth of haematopoietic progenitor cells. Biomed Mater. 2011;6(5):55011.
Tersteeg C, Roest M, Mak-Nienhuis EM, Ligtenberg E, Hoefer IE, Groot PG, et al. A fibronectin-fibrinogen-tropoelastin coating reduces smooth muscle cell growth but improves endothelial cell function. J Cell Mol Med. 2012;16(9):2117–26.
von der Mark K, Park J, Bauer S, Schmuki P. Nanoscale engineering of biomimetic surfaces: cues from the extracellular matrix. Cell Tissue Res. 2010;339(1):131–53.
Hirashima M. Regulation of endothelial cell differentiation and arterial specification by VEGF and Notch signaling. Anat Sci Int. 2009;84(3):95–101.
Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J. 2011;437(2):169–83.
Davies N, Dobner S, Bezuidenhout D, Schmidt C, Beck M, Zisch AH, et al. The dosage dependence of VEGF stimulation on scaffold neovascularisation. Biomaterials. 2008;29(26):3531–8.
Swanson N, et al. In vitro evaluation of vascular endothelial growth factor (VEGF)-eluting stents. Int J Cardiol. 2003;92(2–3):247–51.
Asahara T, Bauters C, Pastore C, Kearney M, Rossow S, Bunting S, et al. Local delivery of vascular endothelial growth factor accelerates reendothelialization and attenuates intimal hyperplasia in balloon-injured rat carotid artery. Circulation. 1995;91(11):2793–801.
van Belle E, Tio FO, Couffinhal T, Maillard L, Passeri J, Isner JM. Stent endothelialization: time course, impact of local catheter delivery, feasibility of recombinant protein administration, and response to cytokine expedition. Circulation. 1997;95(2):438–48.
Byth H, Mchunu BI, Dubery IA, Bornman L. Assessment of a simple, non-toxic alamar blue cell survival assay to monitor tomato cell viability. Phytochem Anal. 2001;12(5):340–6.
Dunkern TR, Kaina B. Cell proliferation and DNA breaks are involved in ultraviolet light-induced apoptosis in nucleotide excision repair-deficient Chinese hamster cells. Mol Biol Cell. 2002;13(1):348–61.
Kamath S, Bhattacharyya D, Padukudru C, Timmons RB, Tang L. Surface chemistry influences implant-mediated host tissue responses. J Biomed Mater Res. 2008;86(3):617–26.
Ertel SI, Ratner BD, Horbett TA. Radiofrequency plasma deposition of oxygen-containing films on polystyrene and poly(ethylene terephthalate) substrates improves endothelial cell growth. J Biomed Mater Res. 1990;24(12):1637–59.
Knetsch ML, Koole LH. VEGF-E enhances endothelialization and inhibits thrombus formation on polymeric surfaces. J Biomed Mater Res. 2010;93(1):77–85.
Wang HG, Yin TY, Ge SP, Zhang Q, Dong QL, Lei DX, et al. Biofunctionalization of titanium surface with multilayer films modified by heparin-VEGF-fibronectin complex to improve endothelial cell proliferation and blood compatibility. J Biomed Mater Res. 2013;101(2):413–20.
Siow KS, Britcher L, Kumar S, Griesser HJ. Plasma methods for the generation of chemically reactive surfaces for biomolecule immobilization and cell colonization—a review. Plasma Process Polym. 2006;3(6–7):392–418.
Pompe T, Keller K, Mothes G, Nitschke M, Teese M, Zimmermann R, et al. Surface modification of poly(hydroxybutyrate) films to control cell-matrix adhesion. Biomaterials. 2007;28(1):28–37.
Yang J, Zeng Y, Zhang C, Chen Y, Yang Z, Li Y, et al. The prevention of restenosis in vivo with a VEGF gene and paclitaxel co-eluting stent. Biomaterials. 2013;34(6):1635–43.
Curcio A, Torella D, Indolfi C. Mechanisms of smooth muscle cell proliferation and endothelial regeneration after vascular injury and stenting: approach to therapy. Circ J. 2011;75(6):1287–96.
Walter DH. Local gene transfer of phVEGF-2 plasmid by gene-eluting stents: an alternative strategy for inhibition of restenosis. Circulation. 2004;110(1):36–45.
Weymann A, Schmack B, Okada T, Soos P, et al. Reendothelialization of human heart valve neoscaffolds using umbilical cord-derived endothelial cells. Circ J. 2013;77(1):207–16.
Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8(2):128–35.
Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schomig A, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370(9591):937–48.
Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, et al. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol. 2007;27(7):1500–10.
van Dyck CJ, Hoymans VY, Haine S, Vrints CJ. New-generation drug-eluting stents: focus on Xience V® everolimus-eluting stent and Resolute® zotarolimus-eluting stent. J Int Cardiol. 2013;26(3):278–86.
Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983;49(3):327–33.
Davies MJ, Woolf N, Rowles PM, Pepper J. Morphology of the endothelium over atherosclerotic plaques in human coronary arteries. Heart. 1988;60(6):459–64.
Jeremy JY, Rowe D, Emsley AM, Newby AC. Nitric oxide and the proliferation of vascular smooth muscle cells. Cardiovasc Res. 1999;43(3):580–94.
Castellot J. Cultured endothelial cells produce heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981;90(2):372–9.
Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989;83(5):1774–7.
Tahir H, Bona-Casas C, Hoekstra AG, Wong KKL. Modelling the effect of a functional endothelium on the development of in-stent restenosis. PLoS One. 2013;8(6):e66138.
Acknowledgments
This work was funded by the German federal government via the BMBF project REMEDIS “Höhere Lebensqualität durch neuartige Mikroimplantate” (FKZ: 03IS2081).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Storm, T., Wulf, K., Teske, M. et al. Chemical activation and changes in surface morphology of poly(ε-caprolactone) modulate VEGF responsiveness of human endothelial cells. J Mater Sci: Mater Med 25, 2003–2015 (2014). https://doi.org/10.1007/s10856-014-5226-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-014-5226-0