Abstract
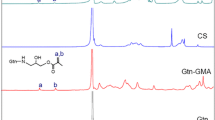
Traditional chitosan hydrogels were prepared by chemical or physical crosslinker, and both of the two kinds of hydrogels have their merits and demerits. In this study, researchers attempted to prepare one kind of chitosan hydrogel by slightly crosslinker, which could combine the advantages of the two kinds of hydrogels. In this experiment, the crosslinker was formed by a reaction between the isocyanate group of 1,6-diisocyanatohexan and the hydroxyl group of polyethylene glycol-400 (PEG-400), then the crosslinker reacted with the amidine and the hydroxyl group of ethylene glycol chitosan to form the network structure. Physical properties of the hydrogel were tested by Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM) and biodegradation. Biocompatibility was assessed by cell implantation in vitro and the scaffold was used as a cartilage tissue engineering scaffold to repair a defect in rabbit knee joints in vivo. FTIR results show the formation of a covalent bond during thickening of the ethylene glycol chitosan. SEM and degradation experiments showed that the ethylene glycol chitosan hydrogel is a 3-D, porous, and degradable scaffold. The hydrogel contained 2 % ethylene glycol chitosan and 10 μl crosslinker was selected for the biocompatibility experiment in vitro and in vivo. After chondrocytes were cultured in the ethylene glycol chitosan hydrogel scaffold for 1 week cells exhibited clustered growth and had generated extracellular matrix on the scaffold in vitro. The results in vivo showed that hydrogel-chondrocytes promoted the repair of defect in rabbits. Based on these results, it could be concluded that ethylene glycol chitosan hydrogel is a scaffold with excellent physicochemical properties and it is a promising tissue engineering scaffold.








Similar content being viewed by others
References
Garg T, Singh O, Arora S, Murthy R. Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst. 2012;29:1–63.
Holzwarth JM, Ma PX. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials. 2011;36:9622–9.
Peng H, Yin Z, Liu H, Chen X, Feng B, Yuan H, et al. Electrospun biomimetic scaffold of hydroxyapatite/chitosan supports enhanced osteogenic differentiation of mMSCs. Nanotechnology. 2012;23:485102.
Amruthwar SS, Janorkar AV. In vitro evaluation of elastin-like polypeptide-collagen composite scaffold for bone tissue engineering. Dent Mater. 2013;29:211–20.
Heiligenstein S, Cucchiarini M, Laschke MW, Bohle RM, Kohn D, Menger MD, et al. In vitro and in vivo characterization of nonbiomedical- and biomedical-grade alginates for articular chondrocyte transplantation. Tissue Eng Part C Methods. 2011;17:829–42.
Jeon O, Powell C, Ahmed SM, Alsberg E. Biodegradable, photocrosslinked alginate hydrogels with independently tailorable physical properties and cell adhesivity. Tissue Eng Part A. 2010;16:2915–25.
Vallet RM, Ruiz HE. Bioceramics: from bone regeneration to cancer nanomedicine. Adv Mater. 2011;23:5177–218.
Sawada Y, Hokugo A, Yang Y, Kamitani M, Matsuda S, Mao T, Lei D, et al. A novel hydroxyapatite ceramic bone substitute transformed by ostrich cancellous bone: characterization and evaluations of bone regeneration activity. J Biomed Mater Res B Appl Biomater. 2011;98B:217–22.
Tsurushima H, Marushima A, Suzuki K, Oyane A, Sogo Y, Nakamura K, et al. Enhanced bone formation using hydroxyapatite ceramic coated with fibroblast growth factor-2. Acta Biomater. 2010;7:2751–9.
Seyednejad H, Gawlitta D, Kuiper RV, de Bruin A, van Nostrum CF, Vermonden T, et al. In vivo biocompatibility and biodegradation of 3D-printed porous scaffolds based on a hydroxyl-functionalized poly (ε-caprolactone). Biomaterials. 2012;33:4309–18.
Zander NE, Orlicki JA, Rawlett AM, Beebe TP Jr. Quantification of protein incorporated into electrospun polycaprolactone tissue engineering scaffolds. Appl Mater Interfaces. 2012;4:2074–81.
Johari N, Fathi MH, Golozar MA, Erfani E, Samadikuchaksaraei A. Poly (ε-caprolactone)/nano fluoridated hydroxyapatite scaffolds for bone tissue engineering: in vitro degradation and biocompatibility study. J Mater Sci Mater Med. 2012;23:763–70.
Ana RC, Duarte JF, Mano RL. Novel 3D scaffold a of chitosan–PLLA blends for tissue engineering applications: Preparation and characterization. J Supercrit Fluids. 2010;3:282.
Huang Y, Onyeri S, Siewe M, Moshfeghian A, Madihally SV. In vitro characterization of chitosan–gelatin scaffolds for tissue engineering. Biomaterials. 2005;26:7616–27.
Kim SE, Suh DH, Yun YP, Lee JY, Park K, Chung JY, et al. Local delivery of alendronate eluting chitosan scaffold can effectively increase osteoblast functions and inhibit osteoclast differentiation. J Mater Sci Mater Med. 2012;23:2739–49.
Coimbra P, Ferreira P, de Sousa HC, Batista P, Rodrigues MA, Correia IJ, et al. Preparation and chemical and biological characterization of a pectin/chitosan polyelectrolyte complex scaffold for possible bone tissue engineering applications. Int J Biol Macromol. 2011;1:112–8.
Cruz DM, Gomes M, Reis RL, Moratal D, Salmerón-Sánchez M, Ribelles JL, et al. Differentiation of mesenchymal stem cells in chitosan scaffolds with double micro and macroporosity. J Biomed Mater Res A. 2010;95:1182–93.
Chen L, Zhu CH, Fan DD, Liu B, Ma XX, Duan ZG, et al. A human-like collagen/chitosan electrospun nanofibrous scaffold from aqueous solution: electrospun mechanism and biocompatibility. J Biomed Mater Res A. 2011;99:395–409.
Frohbergh ME, Katsman A, Botta GP, Lazarovici P, Schauer CL, Wegst UG, et al. Electrospun hydroxyapatite-containing chitosan nanofibers crosslinked with genipin for bone tissue engineering. Biomaterials. 2012;33:9167–78.
Charernsriwilaiwat N, Rojanarata T, Ngawhirunpat T, Opanasopit P. Electrospun chitosan/polyvinyl alcohol nanofibre mats for wound healing. Int Wound J. 2012;11(2):215–22.
Kim S, Nishimoto SK, Bumgardner JD, Haggard WO, Gaber MW, Yang Y. A chitosan/β-glycerophosphate thermo-sensitive gel for the delivery of ellagic acid for the treatment of brain cancer. Biomaterials. 2010;31:4157–66.
Li CY, Ren SG, Dai Y, Tian FJ, Wang X, et al. Efficacy, pharmacokinetics, and biodistribution of thermosensitive chitosan/β-glycerophosphate hydrogel loaded with docetaxel. PharmSciTech. 2014;15:417–24.
Stephanie S, Nicolas A, Nina S, Marc R, Catherine C, et al. Thermosensitive chitosan/glycerophosphate-based hydrogel and its derivatives in pharmaceutical and biomedical applications. Expert Opin Drug Deliv. 2014;11:249–67.
Mohamed AA, Ihab TA. Accelerated wound healing and anti-inflammatory effects of physically cross linked polyvinyl alcohol–chitosan hydrogel containing honey bee venom in diabetic rats. Arch Pharm Res. 2013; 30 (in press).
Nagpal M, Singh SK, Mishra D. Superporous hybrid hydrogels based on polyacrylamide and chitosan: Characterization and in vitro drug release. Int J Pharm Investig. 2013;3:88–94.
Zhang HW, Qadeer A, Mynarcik D, Chen W. Delivery of rosiglitazone from an injectable triple interpenetrating network hydrogel composed of naturally derived materials. Biomaterials. 2011;32:890–8.
Teng DY, Wu ZM, Zhang XG. Synthesis and characterization of in situ cross-linked hydrogel based on self-assembly of thiol-modified chitosan with PEG diacrylate using Michael type addition. Polymer. 2010;3:639–46.
Hu XH, Li D, Gao CY. Chemically cross-linked chitosan hydrogel loaded with gelatin for chondrocyte encapsulation. Biotechnol J. 2011;6:1388–96.
Kuo CK, Ma PX. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials. 2001;22:511–21.
Guo Y, Yuan T, Xiao ZW, Tang PP, Xiao YM, Fan YJ, et al. Hydrogels of collagen/chondroitin sulfate/hyaluronan interpenetrating polymer network for cartilage tissue engineering. J Mater Sci Mater Med. 2012;23:2267–79.
Chiu LL, Reis LA, Radisic M. Controlled delivery of thymosin β-4 for tissue engineering and cardiac regenerative medicine. Ann NY Acad Sci. 2012;1269:16–25.
Wijekoon A, Fountas-Davis N, Leipzig ND. Fluorinated methacrylamide chitosan hydrogel systems as adaptable oxygen carriers for wound healing. Acta Biomater. 2013;9:5653–64.
Zhong X, Ji CD, Chan AK, Kazarian SG, Ruys A, Dehghani F. Fabrication of chitosan/poly (ε-caprolactone) composite hydrogels for tissue engineering applications. J Mater Sci Mater Med. 2011;22:279–88.
Pok S, Myers JD, Madihally SV, Jacot JG. A multilayered scaffold of a chitosan and gelatin hydrogel supported by a PCL core for cardiac tissue engineering. Acta Biomater. 2013;9:5630–42.
Madhumathia K, Shalumona KT, Rania VV, Tamurab WH, Selvamurugana N, Naira SV. Wet chemical synthesis of chitosan hydrogel–hydroxyapatite composite membranes for tissue engineering applications. Int J Biol Macromol. 2009;45:12–5.
Wang Y, Xu N, Luo Q, Li Y, Sun L, et al. In vivo assessment of chitosan/β-glycerophosphate as a new liquid embolic agent. Interv Neuroradiol. 2011;17:87–92.
Hong Y, Song HQ, Gong YH, Mao ZW, Gao CY, Shen JC. Covalently crosslinked chitosan hydrogel: properties of in vitro degradation and chondrocyte encapsulation. Acta Biomater. 2007;3:23–31.
Redman SN, Oldfield SF, Archer CW. Current strategies for articular cartilage repair. Eur Cell Mater. 2005;9:23–32.
Willers C, Partsalis T, Zheng MH. Articular cartilage repair: procedures versus products. Expert Rev Med Devices. 2007;4:373–92.
Hao T, Wen N, Cao JK, Wang HB, Lu SH, Liu T, et al. The support of matrix accumulation and the promotion of sheep articular cartilage defects repair in vivo by chitosan hydrogels. Osteoarthritis Cartilage. 2010;18:257–65.
Acknowledgments
This work was supported by the Natural Science Foundation of China (81171472, 81201407, 81071270).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Z., Zhao, M., Liu, K. et al. Novel chitosan hydrogel formed by ethylene glycol chitosan, 1,6-diisocyanatohexan and polyethylene glycol-400 for tissue engineering scaffold: in vitro and in vivo evaluation. J Mater Sci: Mater Med 25, 1903–1913 (2014). https://doi.org/10.1007/s10856-014-5223-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-014-5223-3