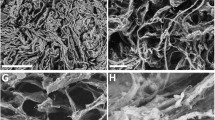
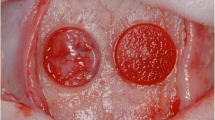
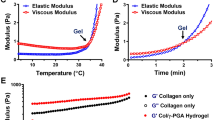
Abstract
Rapid and efficient animal models are needed for evaluating the effectiveness of many new candidate bone regenerative materials. We developed an in vivo model screening for calvarial bone regeneration in lipopolysaccharide (LPS)-treated mice, in which materials were overlaid on the periosteum of the calvaria in a 20 min surgery and results were detectable in 1 week. Intraperitoneal LPS injection reduced spontaneous bone formation, and local application of basic fibroblast growth factor (bFGF) increased the bone-forming activities of osteoblasts. A novel synthetic collagen gel, alkali-treated collagen (AlCol) cross-linked with trisuccinimidyl citrate (TSC), acted as a reservoir for basic substances such as bFGF. The AlCol–TSC gel in conjunction with bFGF activated osteoblast activity without the delay in osteoid maturation caused by bFGF administration alone. The AlCol–TSC gel may slow the release of bFGF to improve the imbalance between osteoid formation and bone mineralization. These findings suggest that our model is suitable for screening bone regenerative materials and that the AlCOl–TSC gel functions as a candidate reservoir for the slow release of bFGF.





Similar content being viewed by others
References
Takagi K, Urist M. The reaction of the dura to bone morphogenetic protein (BMP) in repair of skull defects. Ann Surg. 1982;196:100–9.
Spicer PP, Kretlow JD, Young S, Jansen JA, Kasper FK, Mikos AG. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat Protocols. 2012;7:1918–29.
Lorenzo J, Horowitz M, Choi Y. Osteoimmunology: interactions of the Bone and Immune System. Endocr Rev. 2008;29:403–40.
Taguchi T, Saito H, Uchida Y, Sakane M, Kobayashi H, Kataoka K, et al. Bonding of soft tissues using a novel tissue adhesive consisting of a citric acid derivative and collagen. Mater Sci Eng, C. 2004;24:775–80.
Towler D. Fibroblast growth factor (FGF) and FGF families in bone. In: Bilezikian J, Raisz L, Martin T, editors. Principles of bone biology. 3rd edn., vol 2 San Diego: Academic Press; 2008. p. 1103–1132.
Inoue M, Sasaki M, Nakasu A, Takayanagi M, Taguchi T. An antithrombogenic citric acid-crosslinked gelatin with endothelialization activity. Adv Healthc Mater. 2012;1:573–81.
Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10.
Parfitt A, Drezner M, Glorieux F, Kanis J, Malluche H, Meunier P, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610.
Saito H, Taguchi T, Aoki H, Murabayashi S, Mitamura Y, Tanaka J, et al. pH-responsive swelling behavior of collagen gels prepared by novel crosslinkers based on naturally derived di- or tricarboxylic acids. Acta Biomater. 2007;3:89–94.
Villanueva A, Mathews C, Parfitt A. Relationship between the size and shape of osteoblasts and the width of osteoid seams in bone. In: Takahashi H, editor. Handbook of bone morphometry. 1st ed. Niigata: Nishimura; 1983. p. 191–6.
Morinobu M, Nakamoto T, Hino K, Tsuji K, Shen Z-J, Nakashima K, et al. The nucleocytoplasmic shuttling protein CIZ reduces adult bone mass by inhibiting bone morphogenetic protein-induced bone formation. J Exp Med. 2005;201:961–70.
Kikuchi T, Matsuguchi T, Tsuboi N, Mitani A, Tanaka S, Matsuoka M, et al. Gene expression of osteoclast differentiation factor is induced by lipopolysaccharide in mouse osteoblasts via toll-like receptors. J Immunol. 2001;166:3574–9.
Takami M, Kim N, Rho J, Choi Y. Stimulation by toll-like receptors inhibits osteoclast differentiation. J Immunol. 2002;169:1516–23.
Kawaguchi H, Kurokawa T, Hanada K, Hiyama Y, Tamura M, Ogata E, et al. Stimulation of fracture repair by recombinant human basic fibroblast growth factor in normal and streptozotocin-diabetic rats. Endocrinology. 1994;135:774–81.
Liang H, Pun S, Wronski TJ. Bone anabolic effects of basic fibroblast growth factor in ovariectomized rats. Endocrinology. 1999;140:5780–8.
Kaigler D, Wang Z, Horger K, Mooney D, Krebsbach P. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J Bone Miner Res. 2006;21:735–44.
Mansukhani A, Bellosta P, Sahni M, Basilico C. Signaling by fibroblast growth factors (Fgf) and fibroblast growth factor receptor 2 (Fgfr2)-activating mutations blocks mineralization and induces apoptosis in osteoblasts. J Cell Biol. 2000;149:1297–308.
Tabata Y, Yamada K, Miyamoto S, Nagata I, Kikuchi H, Aoyama I, et al. Bone regeneration by basic fibroblast growth factor complexed with biodegradable hydrogels. Biomaterials. 1998;19:807–15.
Kawaguchi H, Oka H, Jingushi S, Izumi T, Fukunaga M, Sato K, et al. A local application of recombinant human fibroblast growth factor 2 for tibial shaft fractures: a randomized, placebo-controlled trial. J Bone Miner Res. 2010;25:2735–43.
Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–37.
Nagayoshi M, Taguchi T, Koyama H, Takato T, Miyata T, Nagawa H. Enhanced neovascular formation in a novel hydrogel matrix consisting of citric acid and collagen. Ann Vascular Dis. 2011;4:196–203.
Takayama T, Taguchi T, Koyama H, Sakari M, Kamimura W, Takato T, et al. The growth of a vascular network inside a collagen-citric acid derivative hydrogel in rats. Biomaterials. 2009;30:3580–7.
Chikazu D, Taguchi T, Koyama H, Hikiji H, Fujihara H, Suenaga H, et al. Improvement in wound healing by a novel synthetic collage-gel dressing in genetically diabetic mice. Asian J Oral Maxillofac Surg. 2010;22:61–7.
Saito H, Murabayashi S, Mitamura Y, Taguchi T. Unusual cell adhesion and antithrombogenic behavior of citric acid-cross-linked collagen matrices. Biomacromolecules. 2007;8:1992–8.
Ehlers W, Karajan N, Markert B. A porous media model describing the inhomogeneous behaviour of the human intervertebral disc. Materialwiss Werkstofftech. 2006;37:546–51.
Acknowledgments
This study was supported in part by the Industrial Technology Research Grant Program of the New Energy and Industrial Technology Development Organization (NEDO) of Japan. This work was also supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to H.H.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hikiji, H., Tomizuka, K., Taguchi, T. et al. An in vivo murine model for screening cranial bone regenerative materials: testing of a novel synthetic collagen gel. J Mater Sci: Mater Med 25, 1531–1538 (2014). https://doi.org/10.1007/s10856-014-5185-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-014-5185-5