Abstract
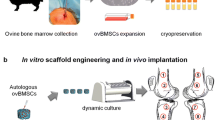
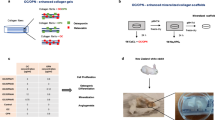
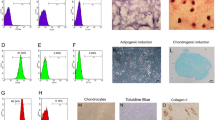
Aseptic loosening in total joint replacements (TJRs) is mainly caused by osteolysis which leads to a reduction of the bone stock necessary for implant fixation in revision TJRs. Our aim was to develop bone tissue-engineered constructs based on scaffolds of clinical relevance in revision TJRs to reconstitute the bone stock at revision operations by using a perfusion bioreactor system (PBRS). The hypothesis was that a PBRS will enhance mesenchymal stem cells (MSCs) proliferation and osteogenic differentiation and will provide an even distribution of MSCs throughout the scaffolds when compared to static cultures. A PBRS was designed and implemented. Scaffolds, silicon substituted hydroxyapatite granules and calcium-phosphate coated porous TiAl6V4 cylinders, were seeded with MSCs and cultured either in static conditions or in the PBRS at 0.75 mL/min. Statistically significant increased cell proliferation and alkaline phosphatase activity was found in samples cultured in the PBRS. Histology revealed a more even cell distribution in the perfused constructs. SEM showed that cells arranged in sheets. Long cytoplasmic processes attached the cells to the scaffolds. We conclude that a novel tissue engineering approach to address the issue of poor bone stock at revision operations is feasible by using a PBRS.






Similar content being viewed by others
References
Harris WH. The problem is osteolysis. Clin Orthop Relat Res. 1995;311:46–53.
Harris WH. Wear and periprosthetic osteolysis. Clin Orthop Relat Res. 2001;393:66–70.
Amstutz HC, Campbell P, Kossovsky N, Clarke IC. Mechanism and clinical significance of wear debris-induced osteolysis. Clin Orthop Relat Res. 1992;276:7–18.
Moore WR, Graves SE, Bain GI. Synthetic bone graft substitutes. ANZ J Surg. 2001;71:354–61.
Vaccaro AR. The role of the osteoconductive scaffold in synthetic bone graft. Orthopaedics. 2002;25:S571–8.
Lanza RP, Langer R, Vacanti J. Principles of tissue engineering. 2nd ed. New York: Academic press; 2000.
Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004;4:743–65.
Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–91.
Ducheyne P, Qiu Q. Bioactive ceramics: the effect of surface reactivity on bone formation and bone cell function. Biomaterials. 1999;20:2287–303.
LeGeros RZ. Calcium phosphate-based osteoinductive materials. Chem Rev. 2008;108:4742–53.
Niinomi M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J Mech Behav Biomed Mater. 2008;1(1):30–42.
Bobyn JD, Stackpool GJ, Hacking SA, et al. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial. J Bone Joint Surg. 1999;81(5):907–14.
Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–50.
Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7.
Ohgushi H, Dohi Y, Tamai S, Tabata S. Osteogenic differentiation of marrow stromal stem cells in porous hydroxyapatite ceramics. J Biomed Mater Res. 1993;27:1401–7.
Oreffo ROC, Driessens FCM, Planell JA, Triffitt JT. Growth and differentiation of human bone marrow osteoprogenitors on novel calcium phosphate cements. Biomaterials. 1998;9:1845–54.
Nishio K, Neo M, Akiyama H, et al. The effect of alkali-and heat-treated titanium and apatite-formed titanium on osteoblastic differentiation of bone marrow cells. J Biomed Mater Res. 2000;52(4):652–61.
García-Gareta E, Hua J, Knowles JC, Blunn GW. Comparison of mesenchymal stem cell proliferation and differentiation between biomimetic and electrochemical coatings on different topographic surfaces. J Mater Sci Mater Med. 2013;24(1):199–210.
Sensebé L, Krampera M, Schrezenmeier H, et al. Mesenchymal stem cells for clinical application. Vox Sang. 2010;98:93–107.
Kale S, Biermann S, Edwards C, et al. Three-dimensional cellular development is essential for ex vivo formation of human bone. Nat Biotechnol. 2000;18:954–8.
Bancroft GN, Sikavitsas VI, Mikos AG. Design of a perfusion bioreactor system for bone tissue-engineering applications. Tissue Eng. 2003;9(3):549–54.
Martin I, Wendt D, Heberer M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004;22(2):80–6.
Sikavitsas V, Bancroft GN, Holtorf HL, et al. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. PNAS. 2003;100(25):14683–8.
Scaglione S, Braccini A, Wendt D, et al. Engineering of osteoinductive grafts by isolation and expansion of ovine bone marrow stromal cells directly on 3D ceramic scaffolds. Biotechnol Bioeng. 2006;93(1):181–7.
Fröhlich M, Grayson WL, Marolt D, et al. Bone grafts engineered from human adipose-derived stem cells in perfusion bioreactor culture. Tissue Eng Part A. 2010;16(1):179–89.
Janssen FW, van Dijkhuizen-Radersma R, Van Oorschot A, et al. Human tissue-engineered bone produced in clinically relevant amounts using a semi-automated perfusion bioreactor system: a preliminary study. J Tissue Eng Regen Med. 2010;4(1):12–24.
Redepenning J, Schlessinger T, Burnham S, et al. Characterization of electrolytically prepared brushite and hydroxyapatite coatings on orthopaedic alloys. J Biomed Mater Res. 1996;30:287–94.
Cartmell SH, Porter BD, Garcia AJ, Guldberg RE. Effects of medium perfusion rate on cell-seeded three-dimensional bone constructs in vitro. Tissue Eng. 2003;9(6):1197–203.
Janssen FW, Oostra J, van Oorschot A, van Blitterswijk CA. A perfusion bioreactor system capable of producing clinically relevant volumes of tissue-engineered bone: in vivo bone formation showing proof of concept. Biomaterials. 2006;27:315–23.
Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312.
Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–94.
Lian JB, Stein GS. Concepts of osteoblast growth and differentiation: basis for modulation of bone cell development and tissue formation. Crit Rev Oral Biol Med. 1992;3(3):269–305.
Hara M, Murakami T, Kobayashi E. In vivo bioimaging using photogenic rats: fate of injected bone marrow-derived mesenchymal stromal cells. J Autoimmun. 2008;30:163–71.
Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–42.
Gie GA, Linder L, Ling RS, et al. Impacted cancellous allografts and cement for revision total hip arthroplasty. J Bone Joint Surg. 1993;75B(1):14–21.
Board TN, Rooney P, Keerney JN, Kay PR. Impaction allografting in revision total hip replacement. J Bone Joint Surg. 2006;88(7):852–7.
Korda M, Blunn G, Goodhip A, Hua J. Use of mesenchymal stem cells to enhance bone formation around revision hip replacements. J Orthop Res. 2008;26:880–5.
Levine BR, Sporer S, Poggie RA, et al. Experimental and clinical performance of porous tantalum in orthopaedic surgery. Biomaterials. 2006;27:4671–81.
Sikavitsas V, Bancroft GN, Lemoine JL, et al. Flow perfusion enhances the calcified matrix deposition of marrow stromal cells in biodegradable nonwoven fiber mesh scaffolds. Ann Biomed Eng. 2005;33(1):63–70.
Bjerre L, Bünger CE, Kassem M, Mygind T. Flow perfusion culture of human mesenchymal stem cells on silicate-substituted tricalcium phosphate scaffolds. Biomaterials. 2008;29:2616–27.
Bancroft GN, Sikavitsas VI, van den Dolder J, et al. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc Natl Acad Sci USA. 2002;99(20):12600–5.
Zhao F, Chella R, Ma T. Effects of shear stress on 3D human mesenchymal stem cell construct development in a perfusion bioreactor system: experiments and hydrodynamic modelling. Biotechnol Bioeng. 2007;96(3):584–95.
Bereither-Hahn J, Münnich A, Woiteneck P. Dependence of energy metabolism on the density of cells in culture. Cell Struct Funct. 1998;23(2):85–93.
Holtorf HI, Sheffield TL, Ambrose CG, et al. Flow perfusion culture of marrow stromal cells seeded on porous biphasic calcium phosphate ceramics. Ann Biomed Eng. 2005;33(9):1238–48.
Mygind T, Stiehler M, Baatrup A, et al. Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials. 2007;28:1036–47.
Dalby MJ, McCloy D, Robertson M, et al. Osteoprogenitor response to semi-ordered and random nanotopographies. Biomaterials. 2006;27:2980–7.
Chatterjea A, Renard AJ, Jolink C, et al. Streamlining the generation of an osteogenic graft by 3D culture of unprocessed bone marrow on ceramic scaffolds. J Tissue Regen Med. 2012;6(2):103–12.
Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87(7):1430–7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
García-Gareta, E., Hua, J., Rayan, F. et al. Stem cell engineered bone with calcium-phosphate coated porous titanium scaffold or silicon hydroxyapatite granules for revision total joint arthroplasty. J Mater Sci: Mater Med 25, 1553–1562 (2014). https://doi.org/10.1007/s10856-014-5170-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-014-5170-z