Abstract
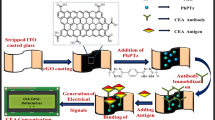
A highly sensitive detection of breast cancer marker, carbohydrate antigen 15-3 (CA 15-3) by carbon nanotube (CNT) based immuno-polymerase chain reaction was reported. The study was aimed to develop a precise and sensitive method to diagnose breast cancer and its recurrence. The hydrofluoric acid (HF) treated silicon wafer layered with bundled CNT was used as the substrate. The surface was treated with HNO3/H2SO4 to graft carboxyl groups on the tips of CNT. Subsequently, polyoxyethylene bis-amine was grafted to conjugate anti human CA 15-3 antibodies. Water contact angle measurement, scanning electron microscope, Fourier transform infrared spectrometer, Raman spectrometer and sodium dodecyl sulfate polyacrylamide gel electrophoresis were employed to confirm the surface modification. The captured antibodies on the CNT were used to capture the target antigen CA 15-3 and the biotinylated secondary antibodies were subsequently bound with the target antigen. A bi-functional streptavidin was used to link biotinylated DNA to the biotinylated detection antibodies. The biotinylated target DNA was amplified by PCR, and then analyzed by agarose gel electrophoresis. The lower limit of detection of CA 15-3 by the proposed immuno-PCR system was 0.001 U/mL, which is extremely sensitive than the other bioanalytical techniques.










Similar content being viewed by others
References
Nima ZA, Mahmood MW, Karmakar A, Mustafa T, Bourdo S, Xu Y, Biris AS. Single-walled carbon nanotubes as specific targeting and Raman spectroscopic agents for detection and discrimination of single human breast cancer cells. J Biomed Opt. 2013;18:55003.
Johnsén C, Bjurstam N. Diagnostic methods in breast cancer. World J Surg. 1977;1:290–4.
Haka AS, Shafer-Peltier KE, Fitzmaurice M, Crowe J, Dasari RR, Feld MS. Diagnosing breast cancer by using Raman spectroscopy. Proc Natl Acad Sci USA. 2005;102:12371–6.
Bedognetti D, Balwit JM, Wang E, Disis ML, Britten CM, Delogu LG, Tomei S, Fox BA, Gajewski TF, Marincola FM, Butterfield LH. SITC/iSBTc cancer immunotherapy biomarkers resource document: online resources and useful tools—a compass in the land of biomarker discovery. J Transl Med. 2011;9:155.
Hanash SM, Pitteri SJ, Faca VM. Mining the plasma proteome for cancer biomarkers. Nature. 2008;452:571–9.
Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nature Rev Cancer. 2005;5:845–56.
Duffy MJ. Serum tumor markers in breast cancer: are they of clinical value? Clin Chem. 2006;52:345–51.
Bon GG, Kenemans P, Verstraeten R, van Kamp GJ, Hilgers J. Serum tumor marker immunoassays in gynecologic oncology: establishment of reference values. Am J Obstet Gynecol. 1996;174:107–14.
Hayes DF, Zurawski VR Jr, Kufe DW. Comparison of circulating CA 15-3 and carcinoembryonic antigen levels in patients with breast cancer. J Clin Oncol. 1986;4:1542–50.
Pons-Anicet DMF, Krebs BP, Namer M. Value of CA 15-3 in the follow-up of breast cancer patients. Br J Cancer. 1987;55:567–9.
Sano T, Smith CL, Cantor CR. Immuno-PCR: very sensitive antigen detection by means of specific antibody-DNA conjugates. Science. 1992;258:120–2.
Adler M, Langer M, Witthohn K, Eck J, Blohm D, Niemeyer CM. Detection of rViscumin in plasma samples by immuno-PCR. Biochem Biophys Res Commun. 2003;300:757–63.
Niemeyer CM, Adler M, Wacker R. Immuno-PCR: high sensitivity detection of proteins by nucleic acid amplification. Trends Biotechnol. 2005;23:208–16.
Joerger RD, Truby TM, Hendrickson ER, Young RM, Ebersole RC. Analyte detection with DNA-labeled antibodies and polymerase chain reaction. Clin Chem. 1995;41:1371–7.
Saito K, Kobayashi D, Sasaki M, Araake H, Kida T, Yagihashi A, Yajima T, Kameshima H, Watanabe N. Detection of human serum tumor necrosis factor-alpha in healthy donors, using a highly sensitive immuno-PCR assay. Clin Chem. 1999;45:665–9.
Wang TW, Lu HY, Lou PJ, Lin FH. Application of highly sensitive, modified glass substrate based immuno-PCR on the early detection of nasopharyngeal carcinoma. Biomaterials. 2008;29:4447–54.
Brakmane G, Madani SY, Seifalian A. Cancer antibody enhanced real time imaging cell probes—a novel theranostic tool using polymer linked carbon nanotubes and quantum dots. Anticancer Agents Med Chem. 2013;13:821–32.
Madani SY, Shabani F, Dwek MV, Seifalian AM. Conjugation of quantum dots on carbon nanotubes for medical diagnosis and treatment. Int J Nanomedicine. 2013;8:941–50.
Shi X, Wang SH, Shen M, Antwerp ME, Chen X, Li C, Petersen EJ, Huang Q, Weber WJ Jr, Baker JR Jr. Multifunctional dendrimer-modified multiwalled carbon nanotubes: synthesis, characterization, and in vitro cancer cell targeting and imaging. Biomacromolecules. 2009;10:1744–50.
Huang SM, Woodson M, Smalley R, Liu J. Growth mechanism of oriented long single walled carbon nanotubes using fast-heating chemical vapor deposition process. Nano Lett. 2004;4:1025–8.
Bianco A, Kostarelos K, Prato M. Applications of carbon nanotubes in drug delivery. Curr Opin Chem Biol. 2005;9:674–9.
De la Zerda A, Zavaleta C, Keren S, Vaithilingam S, Bodapati S, Liu Z, Levi J, Smith BR, Ma TJ, Oralkan O, Cheng Z, Chen X, Dai H, Khuri-Yakub BT, Gambhir SS. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat Nanotechnol. 2008;3:557–62.
Delogu LG, Vidili G, Venturelli E, Ménard-Moyon C, Zoroddu MA, Pilo G, Nicolussi P, Ligios C, Bedognetti D, Sgarrella F, Manetti R, Bianco A. Functionalized multiwalled carbon nanotubes as ultrasound contrast agents. Proc Natl Acad Sci USA. 2012;109:16612–7.
Yi H, Ghosh D, Ham MH, Qi J, Barone PW, Strano MS, Belcher AM. M13 phage-functionalized single-walled carbon nanotubes as nanoprobes for second near-infrared window fluorescence imaging of targeted tumors. Nano Lett. 2012;12:1176–83.
Delogu LG, Stanford SM, Santelli E, Magrini A, Bergamaschi A, Motamedchaboki K, Rosato N, Mustelin T, Bottini N, Bottini M. Carbon nanotube-based nanocarriers: the importance of keeping it clean. J Nanosci Nanotechnol. 2010;10:5293–301.
Hirsch A. Functionalization of single-walled carbon nanotubes. Angew Chem Int Ed Engl. 2002;41:1853–9.
Ghosh S, Dutta S, Gomes E, Carroll D, D’Agostino R Jr, Olson J, Guthold M, Gmeiner WH. Increased heating efficiency and selective thermal ablation of malignant tissue with DNA-encased multiwalled carbon nanotubes. ACS Nano. 2009;3:2667–73.
Lay CL, Liu HQ, Tan HR, Liu Y. Delivery of paclitaxel by physically loading onto poly(ethylene glycol) (PEG)-graft-carbon nanotubes forpotent cancer therapeutics. Nanotechnology. 2010;21:065101.
Nakayama-Ratchford N, Bangsaruntip S, Sun X, Welsher K, Dai H. Noncovalent functionalization of carbon nanotubes by fluorescein-polyethylene glycol: supramolecularconjugates with pH-dependent absorbance and fluorescence. J Am Chem Soc. 2007;129:2448–9.
Delogu LG, Venturelli E, Manetti R, Pinna GA, Carru C, Madeddu R, Murgia L, Sgarrella F, Dumortier H, Bianco A. Ex vivo impact of functionalized carbon nanotubes on human immune cells. Nanomedicine (Lond). 2012;7:231–43.
Pescatori M, Bedognetti D, Venturelli E, Ménard-Moyon C, Bernardini C, Muresu E, Piana A, Maida G, Manetti R, Sgarrella F, Bianco A, Delogu LG. Functionalized carbon nanotubes as immunomodulator systems. Biomaterials. 2013;34:4395–403.
Delogu LG, Magrini A, Bergamaschi A, Rosato N, Dawson MI, Bottini N, Bottini M. Conjugation of antisense oligonucleotides to PEGylated carbon nanotubes enables efficient knockdown of PTPN22 in T lymphocytes. Bioconjugate Chem. 2009;20:427–31.
Zhao B, Yan J, Wang D, Ge Z, He S, He D, Song S, Fan C. Carbon nanotubes multifunctionalized by rolling circle amplification and their application for highly sensitive detection of cancer markers. Small. 2013;. doi:10.1002/smll.201202957.
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5.
Templin MF, Stoll D, Schrenk M, Traub PC, Vöhringer CF, Joos TO. Protein microarray technology. Trends Biotechnol. 2002;20:160–6.
Bange A, Halsall HB, Heineman WR. Microfluidic immunosensor systems. Biosens Bioelectron. 2005;20:2488–503.
Zhang H, Zhao Q, Li XF, Le XC. Ultrasensitive assays for proteins. Analyst. 2007;132:724–37.
Goddard JM, Hotchkiss JH. Polymer surface modification for the attachment of bioactive compounds. Prog Polym Sci. 2007;32:698–725.
Huang TS, Tzeng Y, Liu YK, Chen YC, Walker KR, Guntupalli R, Liu C. Immobilization of antibodies and bacterial binding on nanodiamond and carbon nanotubes for biosensor applications. Diam Relat Mater. 2004;13:1098–102.
Choi YC, Shin YM, Lee YH, Lee BS, Park GS, Choi WB, Lee NS, Kim JM. Controlling the diameter, growth rate, and density of vertically aligned carbon nanotubes synthesized by microwave plasma-enhanced chemical vapor deposition. Appl Phys Lett. 2000;76:2367.
Van Hooijdonk E, Bittencourt C, Snyders R, Colomer JF. Functionalization of vertically aligned carbon nanotubes. Beilstein J Nanotechnol. 2013;4:129–52.
Mirershadi S, Mortazavi SZ, Reyhani A, Moniri N, Novinrooz AJ. Effective condition for purification of multi-walled carbon nanotubes by nitric acid. Synth React Inorg Met Org Nano Met Chem. 2009;39:204–8.
Mazinani S, Ajji A, Dubois C. Morphology, structure and properties of conductive PS/CNT nanocomposite electrospun mat. Polymer. 2009;50:329–42.
Kausaite-Minkstimiene A, Ramanaviciene A, Kirlyte J, Ramanavicius A. Comparative study of random and oriented antibody immobilization techniques on the binding capacity of immunosensor. Anal Chem. 2010;82:6401–8.
Shim M, Kam NWS, Chen RJ, Li Y, Dai H. Functionalization of carbon nanotubes for biocompatibility and biomolecular recognition. Nano Lett. 2002;2:285–8.
Chang CC, Chiu NF, Lin DS, Chu-Su Y, Liang YH, Lin CW. High-sensitivity detection of carbohydrate antigen 15-3 using a gold/zinc oxide thin film surface plasmon resonance-based biosensor. Anal Chem. 2010;82:1207–12.
Zhang HG, Qi C, Wang ZH, Jin G, Xiu RJ. Evaluation of a new CA15-3 protein assay method: optical protein-chip system for clinical application. Clin Chem. 2005;51:1038–40.
Zhang X, Peng X, Jin W. Scanning electrochemical microscopy with enzyme immunoassay of the cancer-related antigen CA15-3. Anal Chim Acta. 2006;558:110–4.
Hong C, Yuan R, Chai Y, Zhuo Y. Ferrocenyl-doped silica nanoparticles as an immobilized affinity support for electrochemical immunoassay of cancer antigen 15-3. Anal Chim Acta. 2009;633:244–9.
Liu YM, Zheng YL, Cao JT, Chen YH, Li FR. Sensitive detection of tumor marker CA15-3 in human serum by capillary electrophoretic immunoassay with chemiluminescence detection. J Sep Sci. 2008;31:1151–5.
He Z, Gao N, Jin W. Capillary electrophoretic enzyme immunoassay with electrochemical detection using a noncompetitive format. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;784:343–50.
Chourb S, Mackness BC, Farris LR, McDonald MJ. Improved detection Of the MUC1 cancer antigen CA 15-3 by ALYGNSA fluorimmunoassay. Health. 2011;3:524–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sadhasivam, S., Chen, JC., Savitha, S. et al. Application of carbon nanotubes layered on silicon wafer for the detection of breast cancer marker carbohydrate antigen 15-3 by immuno-polymerase chain reaction. J Mater Sci: Mater Med 25, 101–111 (2014). https://doi.org/10.1007/s10856-013-5060-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-013-5060-9