Abstract
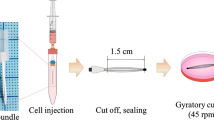
Bioartificial liver support systems are expected to be an effective therapy as a “bridge” for liver transplantation or reversible acute liver disease. A major roadblock in the application of bioartificial livers is the need for a bioreactor that fully meets the requirements of hepatocyte culture, mass transfer and immunobarriers. In this study, we developed a three-dimensional hybrid bioreactor (3DHB) on a base of single-layer skin polyethersulfone hollow fibers by integrating with polyurethane scaffolds. The mass transfer of bilirubin and albumin from the intracapillary space to the extracapillary space of the hollow fibers was not significantly different between 3DHBs and hollow fiber bioreactors (HFBs). Cell viability staining showed that high-density hepatocytes were uniformly found in different regions of the 3DHB after 7 days of culture. Liver-specific functions of human mature hepatocytes cultured in the 3DHB, such as albumin secretion, urea production, ammonia removal rate and cytochrome P450 activity, were maintained stably and were significantly higher compared with the HFB. These results indicated that the 3DHB has good mass transfer and improves cell distribution and liver-specific functions. Meanwhile, the ammonia and unconjugated bilirubin concentrations in plasma from patients with liver failure were significantly decreased during 6 h of circulation by hepatocytes cultured in the 3DHB. Most hepatocytes in the 3DHB were viable after 6 h exposure to the patient plasma. We further demonstrated that bioartificial liver systems with 3DHB can remove toxins from and endure the deleterious effects of the patient plasma. Therefore, the 3DHB has the potential to accomplish different actions for the clinical application of bioartificial livers.







Similar content being viewed by others
References
Demetriou AA, Brown RS, Busuttil RW Jr, et al. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004;239(5):660–7 discussion 67–70.
van de Kerkhove MP, Di Florio E, Scuderi V, et al. Phase I clinical trial with the AMC-bioartificial liver. Int J Artif Organs. 2002;25(10):950–9.
van de Kerkhove MP, Di Florio E, Scuderi V, et al. Bridging a patient with acute liver failure to liver transplantation by the AMC-bioartificial liver. Cell Transplant. 2003;12(6):563–8.
van de Kerkhove MP, Hoekstra R, Chamuleau RA, et al. Clinical application of bioartificial liver support systems. Ann Surg. 2004;240(2):216–30.
Soto-Gutierrez A, Kobayashi N, Rivas-Carrillo JD, et al. Reversal of mouse hepatic failure using an implanted liver-assist device containing ES cell-derived hepatocytes. Nat Biotechnol. 2006;24(11):1412–9.
Si-Tayeb K, Noto FK, Nagaoka M, et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51(1):297–305.
Wang Y, Zhang Y, Zhang S, et al. Rotating microgravity-bioreactor cultivation enhances the hepatic differentiation of mouse embryonic stem cells on biodegradable polymer scaffolds. Tissue Eng Part A. 2012;18(21–22):2376–85.
Zhang S, Chen L, Liu T, et al. Human umbilical cord matrix stem cells efficiently rescue acute liver failure through paracrine effects rather than hepatic differentiation. Tissue Eng Part A. 2012;18(13–14):1352–64.
Strain AJ, Neuberger JM. A bioartificial liver—state of the art. Science. 2002;295(5557):1005–9.
Poyck PP, Pless G, Hoekstra R, et al. In vitro comparison of two bioartificial liver support systems: MELS Cell Module and AMC-BAL. Int J Artif Organs. 2007;30(3):183–91.
Carpentier B, Gautier A, Legallais C. Artificial and bioartificial liver devices: present and future. Gut. 2009;58(12):1690–702.
Allen JW, Hassanein T, Bhatia SN. Advances in bioartificial liver devices. Hepatology. 2001;34(3):447–55.
Davidson AJ, Ellis MJ, Chaudhuri JB. A theoretical method to improve and optimize the design of bioartificial livers. Biotechnol Bioeng. 2010;106(6):980–8.
Kataoka K, Nagao Y, Nukui T, et al. An organic-inorganic hybrid scaffold for the culture of HepG2 cells in a bioreactor. Biomaterials. 2005;26(15):2509–16.
Torok E, Vogel C, Lutgehetmann M, et al. Morphological and functional analysis of rat hepatocyte spheroids generated on poly(l-lactic acid) polymer in a pulsatile flow bioreactor. Tissue Eng. 2006;12(7):1881–90.
Torok E, Lutgehetmann M, Bierwolf J, et al. Primary human hepatocytes on biodegradable poly(l-lactic acid) matrices: a promising model for improving transplantation efficiency with tissue engineering. Liver Transpl. 2011;17(2):104–14.
Miyoshi H, Ehashi T, Kawai H, et al. Three-dimensional perfusion cultures of mouse and pig fetal liver cells in a packed-bed reactor: effect of medium flow rate on cell numbers and hepatic functions. J Biotechnol. 2010;148(4):226–32.
Zhang S, Liu T, Chen L, et al. Bifunctional polyethersulfone hollow fiber with a porous, single-layer skin for use as a bioartificial liver bioreactor. J Mater Sci Mater Med. 2012;23(8):2001–11.
Zhang SC, Liu T, Wang YJ. Porous and single-skinned polyethersulfone membranes support the growth of HepG2 cells: a potential biomaterial for bioartificial liver systems. J Biomater Appl. 2012;27(3):359–66.
Mitry RR. Isolation of human hepatocytes. Methods Mol Biol. 2009;481:17–23.
Lee KD, Kuo TK, Whang-Peng J, et al. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40(6):1275–84.
Hoque ME, Mao HQ, Ramakrishna S. Hybrid braided 3-D scaffold for bioartificial liver assist devices. J Biomater Sci Polym Ed. 2007;18(1):45–58.
De Bartolo L, Salerno S, Curcio E, et al. Human hepatocyte functions in a crossed hollow fiber membrane bioreactor. Biomaterials. 2009;30(13):2531–43.
Monga SP, Hout MS, Baun MJ, et al. Mouse fetal liver cells in artificial capillary beds in three-dimensional four-compartment bioreactors. Am J Pathol. 2005;167(5):1279–92.
Gerlach JC, Schnoy N, Encke J, et al. Improved hepatocyte in vitro maintenance in a culture model with woven multicompartment capillary systems: electron microscopy studies. Hepatology. 1995;22(2):546–52.
Zeilinger K, Holland G, Sauer IM, et al. Time course of primary liver cell reorganization in three-dimensional high-density bioreactors for extracorporeal liver support: an immunohistochemical and ultrastructural study. Tissue Eng. 2004;10(7–8):1113–24.
Bettahalli NM, Vicente J, Moroni L, et al. Integration of hollow fiber membranes improves nutrient supply in three-dimensional tissue constructs. Acta Biomater. 2011;7(9):3312–24.
Miskon A, Yamaoka T, Hyon SH, et al. Preservation of porcine hepatocytes in three-dimensional bioreactor at room temperature using epigallocatechin-3-gallate. Tissue Eng Part C Methods. 2009;15(3):345–53.
Funatsu K, Ijima H, Nakazawa K, et al. Hybrid artificial liver using hepatocyte organoid culture. Artif Organs. 2001;25(3):194–200.
Ran F, Nie S, Zhao W, et al. Biocompatibility of modified polyethersulfone membranes by blending an amphiphilic triblock co-polymer of poly(vinyl pyrrolidone)-b-poly(methyl methacrylate)-b-poly(vinyl pyrrolidone). Acta Biomater. 2011;7(9):3370–81.
Eash HJ, Jones HM, Hattler BG, et al. Evaluation of plasma resistant hollow fiber membranes for artificial lungs. ASAIO J. 2004;50(5):491–7.
Lu HF, Lim WS, Zhang PC, et al. Galactosylated poly(vinylidene difluoride) hollow fiber bioreactor for hepatocyte culture. Tissue Eng. 2005;11(11–12):1667–77.
Torok E, Pollok JM, Ma PX, et al. Optimization of hepatocyte spheroid formation for hepatic tissue engineering on three-dimensional biodegradable polymer within a flow bioreactor prior to implantation. Cells Tissues Organs. 2001;169(1):34–41.
Pahernik SA, Thasler WE, Doser M, et al. High density culturing of porcine hepatocytes immobilized on nonwoven polyurethane-based biomatrices. Cells Tissues Organs. 2001;168(3):170–7.
Yamashita Y, Shimada M, Tsujita E, et al. Polyurethane foam/spheroid culture system using human hepatoblastoma cell line (Hep G2) as a possible new hybrid artificial liver. Cell Transplant. 2001;10(8):717–22.
Sakai Y, Naruse K, Nagashima I, et al. A new bioartificial liver using porcine hepatocyte spheroids in high-cell-density suspension perfusion culture: in vitro performance in synthesized culture medium and in 100 % human plasma. Cell Transplant. 1999;8(5):531–41.
Nibourg GA, Hoekstra R, van der Hoeven TV, et al. Effects of acute-liver-failure-plasma exposure on hepatic functionality of HepaRG-AMC-bioartificial liver. Liver Int. 2012;33(4):516–24.
Nieuwoudt M, Kunnike R, Smuts M, et al. Standardization criteria for an ischemic surgical model of acute hepatic failure in pigs. Biomaterials. 2006;27(20):3836–45.
Hung KC, Yong CC, Chen YS, et al. A surgical model of fulminant hepatic failure in rabbits. Liver Int. 2007;27(10):1333–41.
Li LJ, Du WB, Zhang YM, et al. Evaluation of a bioartificial liver based on a nonwoven fabric bioreactor with porcine hepatocytes in pigs. J Hepatol. 2006;44(2):317–24.
Enosawa S, Miyashita T, Saito T, et al. The significant improvement of survival times and pathological parameters by bioartificial liver with recombinant HepG2 in porcine liver failure model. Cell Transplant. 2006;15(10):873–80.
Chen Z, Ding Y, Xu Q, et al. Bioartificial liver inoculated with porcine hepatocyte spheroids for treatment of canine acute liver failure model. Artif Organs. 2003;27(7):613–22.
Cheng YB, Wang YJ, Zhang SC, et al. Response of porcine hepatocytes in primary culture to plasma from severe viral hepatitis patients. World J Gastroenterol. 2005;11(48):7585–90.
Yamada H, Toda G, Yoshiba M, et al. Humoral inhibitor of rat hepatocyte DNA synthesis from patients with fulminant liver failure. Hepatology. 1994;19(5):1133–40.
Acknowledgments
This research was supported by Grants from the National Natural Science Foundation of China (30800237, 31070880). We gratefully acknowledge Deyuan Science & Technology Development Co., Ltd for preparing the hollow fibers. We thank all members of our laboratory for sharing reagents and advice. We thank American Journal Experts for editorial assistance.
Conflict of interest
The authors indicate no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, S., Chen, L., Liu, T. et al. Integration of single-layer skin hollow fibers and scaffolds develops a three-dimensional hybrid bioreactor for bioartificial livers. J Mater Sci: Mater Med 25, 207–216 (2014). https://doi.org/10.1007/s10856-013-5033-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-013-5033-z