Abstract
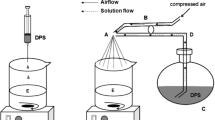
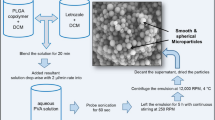
Morphine-loaded poly(l-lactide)-poly(ethylene glycol)-poly(l-lactide) (PLLA-PEG-PLLA) microparticles were prepared using solution enhanced dispersion by supercritical CO2 (SEDS). The effects of process variables on the morphology, particles size, drug loading (DL), encapsulation efficiency and release properties of the microparticles were investigated. All particles showed spherical or ellipsoidal shape with the mean diameter of 2.04–5.73 μm. The highest DL of 17.92 % was obtained when the dosage ratio of morphine to PLLA-PEG-PLLA reached 1:5, and the encapsulation efficiency can be as high as 87.31 % under appropriate conditions. Morphine-loaded PLLA-PEG-PLLA microparticles displayed short-term release with burst release followed by sustained release within days or long-term release lasted for weeks. The degradation test of the particles showed that the degradation rate of PLLA-PEG-PLLA microparticles was faster than that of PLLA microparticles. The results collectively suggest that PLLA-PEG-PLLA can be a promising candidate polymer for the controlled release system.









Similar content being viewed by others
References
Agnihotri SA, Aminabhavi TM. Novel interpenetrating network chitosan-poly(ethylene oxide-g-acrylamide) hydrogel microspheres for the controlled release of capecitabine. Int J Pharm. 2006;324:103–15.
Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17:93–102.
Kawashima Y, York P. Drug delivery applications of supercritical fluid technology. Adv Drug Del Rev. 2008;60:297–8.
Kang Y, Yang C, Ouyang P, Yin G, Huang Z, Yao Y, Liao X. The preparation of BSA-PLLA microparticles in a batch supercritical anti-solvent process. Carbohydr Polym. 2009;77:244–9.
Chen AZ, Li Y, Chau FT, Lau TY, Hu JY, Zhao Z, Mok DKw. Microencapsulation of puerarin nanoparticles by poly(l-lactide) in a supercritical CO2 process. Acta Biomater. 2009;5:2913–9.
Shekunova YB, Baldygab J, York P. Particle formation by mixing with supercritical antisolvent at high Reynolds numbers. Chem Eng Sci. 2001;56:2421–33.
Bristow S, Shekunov T, Shekunov BY, York P. Analysis of the supersaturation and precipitation process with supercritical CO2. J Supercrit Fluids. 2001;21:257–71.
Vert M, Li S, Garreau H. More about the degradation of LA/GA-derived matrices in aqueous media. J Control Release. 1991;16:15–26.
Mothé CG, Drumond WS, Wang SH. Phase behavior of biodegradable amphiphilic poly(l, l-lactide)-b-poly(ethylene glycol)-b-poly(l, l-lactide). Thermochim Acta. 2006;445:61–6.
Dorati R, Genta I, Colonna C, Modena T, Pavanetto F, Perugini P, Conti B. Investigation of the degradation behaviour of poly(ethylene glycol-co-d, l-lactide) copolymer. Polym Degrad Stab. 2007;92:1660–8.
Zhou S, Deng X. In vitro degradation characteristics of poly-dl-lactide–poly(ethylene glycol) microspheres containing human serum albumin. React Funct Polym. 2002;51:93–100.
Park SJ, Kim SH. Preparation and characterization of biodegradable poly(l-lactide)/poly(ethylene glycol) microcapsules containing erythromycin by emulsion solvent evaporation technique. J Colloid Interface Sci. 2004;271:336–41.
Duvvuri S, Janoria KG, Mitra AK. Development of a novel formulation containing poly(d, l-lactide-co-glycolide) microspheres dispersed in PLGA-PEG-PLGA gel for sustained delivery of ganciclovir. J Control Release. 2005;108:282–93.
Hiemstra C, Zhong ZY, Van Tomme SR, Hennink WE, Dijkstra PJ, Feijen J. Protein release from injectable stereocomplexed hydrogels based on PEG-PDLA and PEG-PLLA star block copolymers. J Control Release. 2006;116:e19–21.
Venkatraman SS, Jie P, Min F, Freddy BYC, Leong-Huat G. Micelle-like nanoparticles of PLA-PEG-PLA triblock copolymer as chemotherapeutic carrier. Int J Pharm. 2005;298:219–32.
Andersen G, Christrup L, Sjøgren P. Relationships among morphine metabolism, pain and side effects during long-term treatment: an update. J Pain Symptom Manage. 2003;25:74–91.
Polard E, Le Corre P, Chevanne F, Le Verge R. In vitro and in vivo evaluation of polylactide and polylactide-co-glycolide microspheres of morphine for site-specific delivery. Int J Pharm. 1996;134:37–46.
Moolenaar F, Meyler P, Frijlink E, Jauw TH, Visser J, Proost H. Rectal absorption of morphine from controlled release suppositories. Int J Pharm. 1995;114:117–20.
Eliot L, Butler J, Devane J, Loewen G. Pharmacokinetic evaluation of a sprinkle-dose regimen of a once-daily, extended-release morphine formulation. Clin Ther. 2002;24:260–8.
Portenoy RK, Sciberras A, Eliot L, Loewen G, Butler J, Devane J. Steady-state pharmacokinetic comparison of a new, extended-release, once-daily morphine formulation, Avinza™, and a twice-daily controlled-release morphine formulation in patients with chronic moderate-to-severe pain. J Pain Symptom Manage. 2002;23:292–300.
Alvarez-Fuentes J, Fernández-Arévalo M, Holgado MA, Caraballo I, Rabasco AM, Micó JA, Rojas O, Ortega-Alvaro A. Preclinical study of a controlled release oral morphine system in rats. Int J Pharm. 1996;139:237–41.
Morales ME, Gallardo Lara V, Calpena AC, Doménech J, Ruiz MA. Comparative study of morphine diffusion from sustained release polymeric suspensions. J Control Release. 2004;95:75–81.
Kang Y, Wu J, Yin G, Huang Z, Liao X, Yao Y, Ouyang P, Wang H, Yang Q. Characterization and biological evaluation of paclitaxel-loaded poly(l-lactic acid) microparticles prepared by supercritical CO2. Langmuir. 2008;24:7432–41.
Holgado MA, Iruin A, Alvarez-Fuentes J, Fernández-Arévalo M. Development and in vitro evaluation of a controlled release formulation to produce wide dose interval morphine tablets. Eur J Pharm Biopharm. 2008;70:544–9.
Tozuka Y, Miyazaki Y, Takeuchi H. A combinational supercritical CO2 system for nanoparticle preparation of indomethacin. Int J Pharm. 2010;386:243–8.
Marra F, De Marco I, Reverchon E. Numerical analysis of the characteristic times controlling supercritical antisolvent micronization. Chem Eng Sci. 2012;71:39–45.
Reverchon EM, DE Marco L. Mechanisms controlling supercritical antisolvent precipitate morphology. Chem Eng J. 2011;169:358–70.
Reverchon E, Della Porta G, Sannino D, Ciambelli P. Supercritical antisolvent precipitation of nanoparticles of a zinc oxide precursor. Powder Technol. 1999;102:127–34.
Yang YY, Wan JP, Chung TS, Pallathadka PK, Ng S, Heller J. POE-PEG-POE triblock copolymeric microspheres containing protein: I Preparation and characterization. J Control Release. 2001;75:115–28.
Franceschi E, De Cesaro AM, Feiten M, Ferreira SRS, Dariva C, Kunita MH, Rubira AF, Muniz EC, Corazza ML, Oliveira JV. Precipitation of β-carotene and PHBV and co-precipitation from SEDS technique using supercritical CO2. J Supercrit Fluids. 2008;47:259–69.
Chen AZ, Pu XM, Kang YQ, Liao L, Yao YD, Yin GF. Study of poly(l-lactide) microparticles based on supercritical CO2. J Mater Sci Mater Med. 2007;18:2339–45.
Boutin O, Badens E, Carretier E, Charbit G. Co-precipitation of a herbicide and biodegradable materials by the supercritical anti-solvent technique. J Supercrit Fluids. 2004;31:89–99.
Moshashaée S, Bisrat M, Forbes RT, Nyqvist H, York P. Supercritical fluid processing of proteins: I: lysozyme precipitation from organic solution. Eur J Pharm Sci. 2000;11:239–45.
Alnajjar AO, El-Zaria ME. Synthesis and characterization of novel azo-morphine derivatives for possible use in abused drugs analysis. Eur J Med Chem. 2008;43:357–63.
Sui X, Wei W, Yang L, Zu Y, Zhao C, Zhang L, Yang F, Zhang Z. Preparation, characterization and in vivo assessment of the bioavailability of glycyrrhizic acid microparticles by supercritical anti-solvent process. Int J Pharm. 2012;423:471–9.
Batycky RP, Hanes J, Langer R, Edwards DA. A theoretical model of erosion and macromolecular drug release from biodegrading microspheres. J Pharm Sci. 1997;86:1464–77.
Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Control Release. 2001;73:121–36.
Mallardé D, Boutignon F, Moine F, Barré E, David S, Touchet H, Ferruti P, Deghenghi R. PLGA-PEG microspheres of teverelix: influence of polymer type on microsphere characteristics and on teverelix in vitro release. Int J Pharm. 2003;261:69–80.
Griffith LG. Polymeric biomaterials. Acta Mater. 2000;48:263–77.
Zhao C, Kim SW, Yang DY, Kim JJ, Park NC, Lee SW, Paick JS, Ahn TY, Min KS, Park K, Park JK. Efficacy and safety of once-daily dosing of udenafil in the treatment of erectile dysfunction: results of a multicenter, randomized, double-blind, Placebo-Controlled Trial. Eur Urol. 2011;60:380–7.
Panyam J, Dali MM, Sahoo SK, Ma W, Chakravarthi SS, Amidon GL, Levy RJ, Labhasetwar V. Polymer degradation and in vitro release of a model protein from poly(d, l-lactide-co-glycolide) nano- and microparticles. J Control Release. 2003;92:173–87.
Li S, Garreau H, Vert M. Structure-property relationships in the case of the degradation of massive poly(α-hydroxy acids) in aqueous media. J Mater Sci Mater Med. 1990;1:198–206.
Park TG. Degradation of poly(lactic-co-glycolic acid) microspheres: effect of copolymer composition. Biomaterials. 1995;16:1123–30.
Blanco MD, Sastre RL, Teijón C, Olmo R, Teijón JM. Degradation behaviour of microspheres prepared by spray-drying poly(d, l-lactide) and poly(d, l-lactide-co-glycolide) polymers. Int J Pharm. 2006;326:139–47.
Gopferich A, Langer R. Modeling of polymer erosion. Macromolecules. 1993;26:4105–12.
Youxin L, Volland C, Kissel T. In-vitro degradation and bovine serum albumin release of the ABA triblock copolymers consisting of poly (l(+) lactic acid), or poly(l(+) lactic acid-co-glycolic acid) A-blocks attached to central polyoxyethylene B-blocks. J Control Release. 1994;32:121–8.
Acknowledgments
This work has been supported by the National Natural Science Foundation of China (project No. 51173120, 51273122 and 51202151). Authors are very much grateful to the National Engineering Research Center for Biomaterials, Sichuan University for the assistance with the microscopy work.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, F., Yin, G., Liao, X. et al. Preparation, characterization and in vitro release properties of morphine-loaded PLLA-PEG-PLLA microparticles via solution enhanced dispersion by supercritical fluids. J Mater Sci: Mater Med 24, 1693–1705 (2013). https://doi.org/10.1007/s10856-013-4926-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-013-4926-1