Abstract
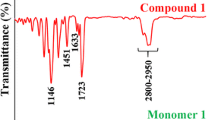
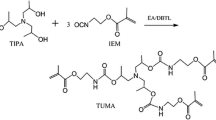
A novel tertiary amine containing urethane dimethacrylate monomer UDMTA was synthesized with the aim of replacing Bis-GMA as one component of dental restorative materials. The structure of UDMTA was confirmed by FT-IR and 1H-NMR spectra. UDMTA was incorporated into Bis-GMA/TEGDMA (50 wt%/50 wt%) resin system to replace Bis-GMA partly and totally. Double bond conversion, polymerization volumetric shrinkage, water sorption and solubility, flexural strength and modulus of UDMTA containing resin formulations were studied with neat Bis-GMA/TEGDMA resin formulation as a reference. Results showed that UDMTA could be used as a coinitiator in photocurable dental resin, UDMTA containing resin had higher double bond conversion and lower polymerization shrinkage than that of Bis-GMA/TEGDMA resin, and the UDMTA containing copolymer had higher flexural strength and flexural modulus than Bis-GMA/TEGDMA copolymer. When UDMTA was used to replace more than 25 wt% of Bis-GMA, the obtained copolymer had higher water sorption and solubility. The optimized resin composition is by replacing 25 wt% of Bis-GMA in Bis-GMA/TEGDMA (50/50 by wt%), for the prepared resin had the best comprehensive properties.






Similar content being viewed by others
References
Vallittu PK, Miettinen V, Alakuijala P. Residual monomer content and its release into water from denture base materials. Dent Mater. 1995;11:338–42.
Narva KK, Lassila LV, Vallittu PK. The static strength and modulus of fiber reinforced denture base polymer. Dent Mater. 2005;21:421–8.
Viljanen EK, Lassila LV, Skrifvars M, Vallittu PK. Degree of conversion and flexural properties of a dendrimer/methyl methacrylate copolymer: design of experiments and statistical screening. Dent Mater. 2005;21:172–7.
Viljanen EK, Skrifvars M, Vallittu PK. Dendritic copolymers and particulate filler composites for dental applications: degree of conversion and thermal properties. Dent Mater. 2007;23:1420–7.
He J, Luo Y, Liu F, Jia D. Synthesis and characterization of a new trimethacrylate monomer with low polymerization shrinkage and its application in dental restoration materials. J Biomater Appl. 2010;25:235–49.
Wang T, Nikaido T, Nakabayashi N. Photocure bonding agent containing phosphoric methacrylate. Dent Mater. 1991;7:59–62.
Munksgaard EC. Permeability of protective gloves by HEMA and TEGDMA in the presence of solvents. Acta Odontol Scand. 2000;58:57–62.
Liu F, He JW, Lin ZM, Ling JQ, Jia DM. Synthesis and characterization of dimethacrylate monomer with high molecular weight for root canal filling materials. Molecules. 2006;11:953–8.
Lin ZM, Ling JQ, Fang JY, Liu F, He JW. Physicochemical properties, sealing ability, bonding strength and cytotoxicity of a new dimethacrylate-based root canal sealer. J Formos Med Assoc. 2010;109:819–27.
He J, Luo Y, Liu F, Jia D. Synthesis, characterization and photopolymerization of a new dimethacrylate monomer based (alpha-methyl-benzylidene)-bisphenol used as root canal sealer. J Biomater Sci Polym Ed. 2010;21:1191–205.
Kim YK, Grandini S, Ames JM, Gu LS, Kim SK, Pashley DH, et al. Critical review on methacrylate resin-based root canal sealer. J Endod. 2010;36:383–99.
Chung CM, Kim MS, Kim JG, Jang DO. Synthesis and photopolymerization of trifunctional methacrylates and their application as dental monomers. J Biomed Mater Res. 2002;62:622–7.
Barszczewska-Rybarek IM. Structure–property relationships in dimethacrylate networks based on Bis-GMA, UDMA and TEGDMA. Dent Mater. 2009;25:1082–9.
Moszner N, Salz U. New developments of polymeric dental composites. Prog Polym Sci. 2001;26:535–76.
Ellakwa A, Cho N, Lee IB. The effect of resin matrix composition on the polymerization shrinkage and rheological properties of experimental dental composites. Dent Mater. 2007;23:1229–35.
Nie J, Wu G. 3-diethylamino-propionate methacrylate as a polymerization amine coinitiator for dental application. Dent Mater. 2007;23:623–9.
Viljanen EK, Skrifvars M, Vallittu PK. Dendrimer/methyl methacrylate copolymers: residual methyl methacrylate and degree of conversion. J Biomater Sci Polym Ed. 2005;16:1219–31.
Viljanen EK, Langer S, Skrifvars M, Vallittu PK. Analysis of residual monomers by HPLC and HS-GC/MS. Dent Mater. 2006;22:845–51.
Sideridou I, Achilias DS, Spyroudi C, Karabela M. Water sorption characteristics of light-cured dental resins and composites based on Bis-EMA/PCDMA. Biomaterials. 2004;25:367–76.
Khatri CA, Stansbury JW, Schultheisz CR, Antonucci JM. Synthesis, characterization and evaluation of urethane derivatives of Bis-GMA. Dent Mater. 2003;19:584–8.
Kim JG, Chung CM. Trifunctional methacrylate monomers and their photocured composites with reduced curing shrinkage, water sorption, and water solubility. Biomaterials. 2003;24:3845–51.
Kurata S, Yamazaki N. Synthesis of dimethacryloxy ethyl-1,1,6,6-tetrahydro- perflourohexamethylene-1,6-dicarbamate as dental base monomers and the mechanical properties of the copolymers of the monomer and methyl methacrylate. Dent Mater J. 2011;30:103–8.
Huang YQ, Wong CKC, Zheng JS, Bouwman H, Barra R, Wahlström B, et al. Bisphenol A (BPA) in China: a review of sources, environmental levels, and potential human health impacts. Environ Int. 2012;42:91–9.
International Standardization Organization ISO 10477 1992(E). Dentistry-polymer based crown and bridge materials. Geneva, Switzerland: International Standardization Organization; 1992.
Xu H, Wu G, Nie J. Synthesis and photopolymerization characteristics of amine coinitiator. J Photochem Photobiol, A. 2008;193:254–9.
Schneider LFJ, Cavalcante LM, Consani S, Ferracane JL. Effect of co-initiator ratio on the polymer properties of experimental resin composites formulated with camphorquinone and phenyl-propanedione. Dent Mater. 2009;25:369–75.
He J, Liu F, Luo Y, Jia D. Synthesis and characterization of a dimethacrylates monomer with low shrinkage and water sorption for dental application. J Appl Polym Sci. 2012;125:114–20.
Venhoven BAM, de Gee AJ, Davidson CL. Polymerization contraction and conversion of light-curing Bis-GMA-based methacrylate resins. Biomaterials. 1993;14:871–5.
Patel MP, Braden M, Davy KWM. Polymerization shrinkage of methacrylate esters. Biomaterials. 1987;8:53–6.
Magali D, Belphine TB, Jacques D, Gäetane L. Volume contaction in photocured dental resins: the shrinkage-conversion relationship revisited. Dent Mater. 2006;22:359–65.
Ge J, Trujillo M, Stansbury J. Synthesis and photopolymerization of low shrinkage methacrylate monomers containing bulky substituent groups. Dent Mater. 2005;21:1163–9.
Stansbury JW, Trujillo-Lemon M, Lu H, Ding X, Lin Y, Ge J. Conversion-dependent shrinkage stress and strain in dental resins and composites. Dent Mater. 2005;21:56–67.
Sideridou ID, Karabela MM, Vouvoudi EC. Dynamic thermomechanical properties and sorption characteristics of two commercial light cured dental resin composites. Dent Mater. 2008;24:737–43.
Ferracane JL, Condon JR. Rate of elution of leachable components from composite. Dent Mater. 1990;6:282–7.
Soderholm KJ, Zigan M, Ragan M, Fischlshweiger W, Bergman M. Hydrolytic degradation of dental composites. J Dent Res. 1984;63:1248–54.
Costella AM, Trochmann JL, Oliveira WS. Water sorption and diffusion coefficient through an experimental dental resin. J Mater Sci Mater Med. 2010;21:67–72.
Puffer R, Sebenda J. On the structure and properties of polyamides XXVII. The mechanism of water sorption in polyamide. J Polym Sci C. 1967;16:79.
Lastumäki T, Lassila L, Vallittu PK. Flexural properties of bulk fiber-reinforced composite DC-Tell used in fixed partial dentures. Int J Prosthodont. 2001;14:22–6.
Sideridou I, Tserki V, Papanastasiou G. Study of water sorption, solubility and modulus of elasticity of light-cured dimethacrylate-based dental resins. Biomaterials. 2003;24:655–65.
Chang MC, Lin LD, Chuang FH, Chan CP, Wang TM, Lee JJ, et al. Carboxylesterase expression in human dental pulp cells: role in regulation of BisGMA-induced prostanoid production and cytotoxicity. Acta Biomater. 2012;8:1380–7.
Hansel C, Leyhause G, Mai UE, Geurtsen W. Effects of various resin composite (co)monomers and extracts on two caries-associated micro-organisms in vitro. J Dent Res. 1998;77:60–7.
Ferracane JL. Cytotoxicity of components of resins and other dental restorative materials. J Oral Rehabil. 1994;21:453–62.
Asmussen E, Peutzfeldt A. Influence of UEDMA, BisGMA and TEGDMA on selected mechanical properties of experimental resin composites. Dent Mater. 1998;14:51–6.
Acknowledgments
The study was supported by the Fundamental Research Funds for the Central Universities (2012ZB0004), China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, D., Liu, F., He, J. et al. Synthesis of a novel tertiary amine containing urethane dimethacrylate monomer (UDMTA) and its application in dental resin. J Mater Sci: Mater Med 24, 1595–1603 (2013). https://doi.org/10.1007/s10856-013-4897-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-013-4897-2