Abstract
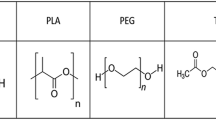
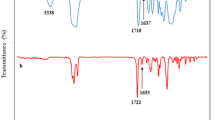
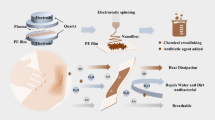
Preparation and evaluation of new polyurethane membranes for wound dressing application was considered in this work. The membranes were prepared through amine curing reaction of epoxy-terminated polyurethane prepolymers and an antibacterial epoxy-functional quaternary ammonium compound (glycidyltriehtylammonium chloride, GTEACl. To render the prepared membranes to be highly absorptive of wound exudates, poly (ethylene glycol) polyols were introduced into the polyurethane networks. Evaluation of biocompatibity via both MTT assay and direct contact with two different cell lines (fibroblast and epidermal keratinocytes) reveled that membranes with appropriate loading of GTEACl showed proper biocompatibility. Promising antibacterial activity of the prepared membranes against Staphylococcus aureus and Escherichia coli bacteria was confirmed by both agar diffusion and shaking flask methods. The membranes with balanced crosslink density and ionic groups’ concentration possessed appropriate hydrophilicity and water vapor transmission rate; therefore, they could prevent the accumulation of exudates and decrease the surface inflammation in the wounded area.









Similar content being viewed by others
References
Elias PM. The skin barrier as an innate immune element. Semin Immunopathol. 2007;29:3–14.
Pivarcsi A, Nagy I, Kemeny L. Innate immunity in the skin: how keratinocytes fight against pathogens. Curr Immunol Rev. 2005;1:29–42.
Jayakumar R, Prabaharan M, Kumar PTS, Nair SV, Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv. 2011;29:322–37.
Aramwit P, Muangman P, Namviriyachote N, Srichana T. In vitro evaluation of the antimicrobial effectiveness and moisture binding properties of wound dressings. Int J Mol Sci. 2010;11:2864–74.
Ignacio C, Barcellos L, Ferreira MD, Moura SAL, Soares IA, Oréfice RL. In vivo tests of a novel wound dressing based on biomaterials with tissue adhesion controlled through external stimuli. J Mater Sci Mater Med. 2011;22:1357–64.
Rossum Mv, Vooijs DPP, Walboomers XF, Hoekstra MJ, Spauwen PHM, Jansen JA. The influence of a PHI-5-loaded silicone membrane, on cutaneous wound healing in vivo. J Mater Sci Mater Med. 2007;18:1449–56.
Lionelli GT, Lawrence WT. Wound dressings. Surg Clin N Am. 2003;83:617–38.
Yoo H-J, Kim H-D. Synthesis and properties of waterborne polyurethane hydrogels for wound healing dressings. J Biomed Mater Res Part B. 2008;85B:326–33.
Witthayaprapakorn C, Molloy R, Nalampang K, Tighe BJ. Design and preparation of a bioresponsive hydrogel for biomedical application as a wound dressing. Adv Mater Res. 2008;55–57:681–4.
Pal K, Banthia AK, Majumdar DK. Biomedical evaluation of polyvinyl alcohol-gelatin esterified hydrogel for wound dressing. J Mater Sci Mater Med. 2007;18:1889–94.
Peles Z, Zilberman M. Novel soy protein wound dressings with controlled antibiotic release: mechanical and physical properties. Acta Biomater. 2012;8:209–17.
Chen J-P, Chiang Y. Bioactive electrospun silver nanoparticles-containing polyurethane nanofibers as wound dressings. J Nanosci Nanotechnol. 2010;10:7560–4.
Peng HT, Martineau L, Hung A. Hydrogel-elastomer composite biomaterials: 4. experimental optimization of hydrogel-elastomer composite fibers for use as a wound dressing. J Mater Sci Mater Med. 2008;19:1803–13.
Mertz PM, Marshall DA, Eaglstern WH. Occlusive wound dressings to prevent bacterial invasion and wound infection. J Am Acad Dermatol. 1985;12:662–8.
Mayer D, Tsapogas M. Povidone–iodine and wound healing: a critical review. Wounds. 1993;5:14–23.
Mertz PM, Marshall DA, Kuglar MA. Povidone–iodine in polyethylene oxide hydrogel dressing: effect on multiplication of Staphylococcus aureus in partial thickness wounds. Arch Dermatol. 1986;122:1133–8.
Mandy SH. Evaluation of a new povidone–iodine-impregnated polyethylene oxide gel occlusive dressing. J Am Acad Dermatol. 1985;13:655–9.
Dizman B, Elasri MΟ, Mathias LJ. Novel antibacterial polymers. Smart Coatings II, ACS symposium series, vol 1002. Washington: American Chemical Society; 2009. p. 27–50.
Kenawy E-R, Worley SD, Broughton R. The chemistry and applications of antimicrobial polymers: a state-of-the-art review. Biomacromolecules. 2007;8(5):1359–84. doi:10.1021/bm061150q.
Vasilev K, Cook J, Griesser HJ. Antibacterial surfaces for biomedical devices. Expert Rev Med Devices. 2009;6:553–67.
Stratton TR, Rickus JL, Youngblood JP. In vitro biocompatibility studies of antibacterial quaternary polymers. Biomacromolecules. 2009;10:2550–5.
Harney MB, Pant RR, Fulmer PA, Wynne JH. Surface self-concentrating amphiphilic quaternary ammonium biocides as coating additives. Appl Mater Interfaces. 2009;1:39–41.
Yao C, Li X, Neoh KG, Shi Z, Kang ET. Surface modification and antibacterial activity of electrospun polyurethane fibrous membranes with quaternary ammonium moieties. J Membr Sci. 2008;320:259–67.
Sajomsang W, Gonil P, Tantayanon S. Antibacterial activity of quaternary ammonium chitosan containing mono or disaccharide moieties: preparation and characterization. Int J Biol Macromol. 2009;44:419–27.
Alipour SM, Nouri M, Mokhtari J, Bahrami SH. Electrospinning of poly (vinyl alcohol)–water-soluble quaternized chitosan derivative blend. Carbohydr Res. 2009;344:2496–501.
Yang JM, Yang SJ, Lin HT, Wu T-H, Chen H-J. Chitosan containing PU/Poly (NIPAAm) thermosensitive membrane for wound dressing. Mater Sci Eng C. 2008;28:150–6.
Thomas V, Muthu J. Biomechanical studies on aliphatic physically crosslinked poly (urethane urea) for blood contact applications. J Mater Sci Mater Med. 2008;19:2721–33.
Yeganeh H, Orang F, Solouk A, Rafienia M. Synthesis, characterization and preliminary investigation of blood compatibility of novel epoxy-modified polyurethane networks. J Bioact Compat Polym. 2008;23:276–300.
Yeganeh H, Lakouraj MM, Jamshidi S. Synthesis and characterization of novel biodegradable epoxy-modified polyurethane elastomers. J Poylm Sci Part A. 2005;43:2985–96.
Mcclure JD. Glycidyltrimethylammonium chloride and related compounds. J Org Chem. 1970;35:2059–61.
Yeganeh H, Hojati-Talemi P. Preparation and properties of novel biodegradable polyurethane networks based on castor oil and poly (ethylene glycol). Polym Degrad Stab. 2007;92:480–9.
Lee H, Neville K. Handbook of Epoxy Resin. New York: McGraw-Hill Co.; 1967.
Yagci MB, Bolca S, Heuts JPA, Ming W, With Gd. Antimicrobial polyurethane coatings based on ionic liquid quaternary ammonium compounds. Prog Org Coat. 2011;72:343–7.
Wynne JH, Fulmer PA, McCluskey M, Mackey NM, Buchanan JP. Synthesis and development of a multifunctional self-decontaminating polyurethane coating. ACS Appl Mater Interfaces. 2011;3:2005–11.
Krone CA, Ely JTA, Klingner T, Rando RJ. Isocyanates in flexible polyurethane foams. Bull Environ Contam Toxicol. 2003;70:328–35.
Daman AP, Jickells SM, Castle L. Liquid chromatographic determination of residual isocyanate monomers in plastics intended for food contact use. J AOAC Int. 1995;78:711–9.
Duff DW, Maciel GE. Monitoring postcure reaction chemistry of residual isocyanate in 4,4′-methylene bis (phenyl isocyanate) based isocyanurate resins by I5N and I3C CP/MAS NMR. Macromolecules. 1991;24:381–97.
Yeganeh H, Jamshidi H, Jamshidi S. Synthesis and properties of novel biodegradable poly(ε-caprolactone)/poly (ethylene glycol) based polyurethane elastomers. Polym Int. 2007;56:41–9.
Yeganeh H, Lakouraj MM, Jamshidi S. Synthesis and properties of biodegradable elastomeric epoxy modified polyurethanes based on poly (ε-caprolactone) and poly(ethylene glycol). Eur Polym J. 2005;41:2370–9.
Chattopadhyay DK, Zakula AD, Dean Webster C. Organic-inorganic hybrid coatings prepared from glycidyl carbamate resin, 3-aminopropyl trimethoxy silane and tetraethoxyorthosilicate. Prog Org Coat. 2009;64:128–37.
Edwards PA, Striemer G, Webster DC. Synthesis, characterization and self-crosslinking of glycidyl carbamate functional resins. Prog Org Coat. 2006;57:128–39.
Dietrich D, Keberle W, Witt H. Polyurethane ionomers, a new class of block polymers. Anpew Chem Int Ed Engl. 1970;9:40–50.
Goddard RJ, Cooper SL. Polyurethane cationomers with pendant trimethylammonium groups. 1. Fourier transform infrared temperature studies. Macromolecules. 1995;28:1390–400.
Khranovskii VA, Lipatov YS, Maslyuk AF. Dokl Akad Nauk SSSR Ser A. 1985;285:406–10.
Reynolds N, Spiess HW, Hayen H, Nefzger H, Eisenbach CD. Structure and deformation behaviour of model poly (ether–urethane) elastomers, 1. Infrared studies. Macromol Chem Phys. 1994;195:2855–73.
Wilkes CE, Yusek CS. Investigation of domain structure in urethan elastomers by X-ray and thermal methods. J Macromol Sci Phys. 1973;B7:157–75.
Niesten MCEJ, Brinke ten JW, Gaymans RJ. Segmented copolyetheresteraramids with extended poly (tetramethyleneoxide) segments. Polymer. 2001;42:1461–9.
Christenson EM, Anderson JM, Hiltner A, Baer E. Relationship between nanoscale deformation processes and elastic behavior of polyurethane elastomers. Polymer. 2005;46:11744–54.
Queen D, Gaylor JDS, Ebans JH, Courtney JM. The preclinical evaluation of the water vapour transmission rate through burn wound dressings. Biomaterials. 1987;8:367–71.
Eaglstein WH, Davis SC, Mehle AL, Mertz PM. Optimal use of an occlusive dressing to enhance healing—effect of delayed application and early removal on wound healing. Arch Dermatol. 1988;124:392–5.
Atiyeh BS, Ioannovich J, Al-Amm CA, El-Musa KA. Management of acute and chronic open wounds: The importance of moist environment in optimal wound healing. Curr Pharm Biotechnol. 2002;3:179–95.
Field CK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167:S2–6.
Zhao L, Xu L, Mitomo H, Yoshii F, Polymers C. Synthesis of pH-sensitive PVP/CM-chitosan hydrogels with improved surface property by irradiation. Carbohydr Polym. 2006;64:473–80.
Chen J-H, Ruckenstein E. Solvent-stimulated surface rearrangement of polyurethanes. J Colloid Interface Sci. 1990;135:496–507.
Balakrishnan B, Mohanty M, Umashankarc PR, Jayakrishnan A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials. 2005;26:6335–42.
Nagamune H, Maeda T, Ohkura K, Yamamoto K, Nakajima M, Kourai H. Evaluation of the cytotoxic effects of bis-quaternary ammonium antimicrobial reagents on human cells. Toxicol In Vitro. 2000;14:139–47.
Ikeda T, Hirayama H, Yamaguchi H, Tazuke S, Watanabe M. Polycationic biocides with pendant active groups: molecular weight dependence of antibacterial activity. Antimicrob Agents Chemother. 1986;30:132–6.
Pasquier N, Keul H, Heine E, Moeller M. From multifunctionalized poly (ethylene imine)s toward antimicrobial coatings. Biomacromolecules. 2007;8:2874–82.
Zhu Y, Hu J, Yeung K. Effect of soft segment crystallization and hard segment physical crosslink on shape memory function in antibacterial segmented polyurethane ionomers. Acta Biomater. 2009;5:3346–57.
Westman EH, Ek M, Enarsson LE, Wagberg L. Assessment of antibacterial properties of polyvinylamine (PVAm) with different charge densities and hydrophobic modifications. Biomacromolecules. 2009;10:1478–83.
Huang J, Murata H, Koepsel RR, Russell AJ, Matyjaszewski K. Antibacterial polypropylene via surface-initiated atom transfer radical polymerization. Biomacromolecules. 2007;8:1396–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yari, A., Yeganeh, H. & Bakhshi, H. Synthesis and evaluation of novel absorptive and antibacterial polyurethane membranes as wound dressing. J Mater Sci: Mater Med 23, 2187–2202 (2012). https://doi.org/10.1007/s10856-012-4683-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-012-4683-6