Abstract
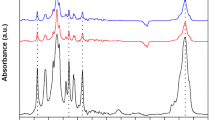
Bionanocomposite scaffolds comprised of nanomaterials and the extracellular matrix (ECM) of porcine diaphragm tissue capitalizes on the benefits of utilizing a natural ECM material, while also potentially enhancing physicomechanical properties and biocompatibility through nanomaterials. Gold nanoparticle (AuNP) bionanocomposite scaffolds were subjected to a number of characterization techniques to determine whether the fabrication process negatively impacted the properties of the porcine diaphragm tissue and whether the AuNP improved the properties of the tissue. Tensile testing and differential scanning calorimetry demonstrated that the bionanocomposite possessed improved tensile strength and thermal stability relative to natural tissue. The collagenase assay and Fourier transform infrared spectroscopy additionally confirmed that denaturation of the collagen of the ECM did not occur. The novel bionanocomposite scaffold possessed properties similar to commercially available scaffolds and will be further developed for soft tissue applications such as hernia repair through in vivo studies in an animal model.




Similar content being viewed by others
References
Hsu SH, Tang CM, Tseng HJ. Biocompatibility of poly(ether)urethane-gold nanocomposites. J Biomed Mater Res A. 2006;79:759–70.
Wu ZS, Zhang SB, Guo MM, Chen CR, Shen GL, Yu RQ. Homogeneous, unmodified gold nanoparticle-based colorimetric assay of hydrogen peroxide. Anal Chim Acta. 2007;584:122–8.
Chou CW, Hsu SH, Wang PH. Biostability and biocompatibility of poly(ether)urethane containing gold or silver nanoparticles in a porcine model. J Biomed Mater Res A. 2008;84:785–94.
Owens DE III, Eby JK, Jian Y, Peppas NA. Temperature-responsive polymer-gold nanocomposites as intelligent therapeutic systems. J Biomed Mater Res A. 2007;83:692–5.
O’Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004;209:171–6.
Hsu S, Chou C, Tseng S. Enhanced thermal and mechanical properties in polyurethane/Au nanocomposites. Macromol Mater Eng. 2004;289:1096–101.
Lin YL, Jen JC, Hsu SH, Chiu IM. Sciatic nerve repair by microgrooved nerve conduits made of chitosan-gold nanocomposites. Surg Neurol. 2008. doi:10.1016/j.surneu.2008.01.057.
Niidome T, Yamagata M, Okamoto Y, Akiyama Y, Takahashi H, Kawano T, Katayama Y, Niidome Y. PEG-modified gold nanorods with a stealth character for in vivo applications. J Control Release. 2006;114:343–7.
Sershen SR, Westcott SL, Halas NJ, West JL. Temperature-sensitive polymer-nanoshell composites for photothermally modulated drug delivery. J Biomed Mater Res. 2000;51:293–8.
Pingarron J, Yanez-Sedeno P, Gonzalez-Cortes A. Gold nanoparticle-based electrochemical biosensors. Electrochim Acta. 2008;53:5848–66.
Hillyer JF, Albrecht RM. Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J Pharm Sci. 2001;90:1927–36.
Alric C, Taleb J, Le DG, Mandon C, Billotey C, Le Meur-Herland A, Brochard T, Vocanson F, Janier M, Perriat P, Roux S, Tillement O. Gadolinium chelate coated gold nanoparticles as contrast agents for both X-ray computed tomography and magnetic resonance imaging. J Am Chem Soc. 2008;130:5908–15.
Everts M, Saini V, Leddon JL, Kok RJ, Stoff-Khalili M, Preuss MA, Millican CL, Perkins G, Brown JM, Bagaria H, Nikles DE, Johnson DT, Zharov VP, Curiel DT. Covalently linked Au nanoparticles to a viral vector: potential for combined photothermal and gene cancer therapy. Nano Lett. 2006;6:587–91.
Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104:293–346.
Haidekker MA, Boettcher LW, Suter JD, Rone R, Grant SA. Influence of gold nanoparticles on collagen fibril morphology quantified using transmission electron microscopy and image analysis. BMC Med Imaging. 2006;6:4–10.
Xie J, Macewan MR, Li X, Sakiyama-Elbert SE, Xia Y. Neurite outgrowth on nanofiber scaffolds with different orders, structures, and surface properties. ACS Nano. 2009;3:1151–9.
Xie J, Macewan MR, Ray WZ, Liu W, Siewe DY, Xia Y. Radially aligned, electrospun nanofibers as dural substitutes for wound closure and tissue regeneration applications. ACS Nano. 2010;4:5027–36.
Badylak SF. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol. 2004;12:367–77.
Hodde J. Naturally occurring scaffolds for soft tissue repair and regeneration. Tissue Eng. 2002;8:295–308.
Deeken CR, White AK, Bachman SL, Ramshaw BJ, Cleveland DS, Loy TS, Grant SA. Method of preparing a decellularized porcine tendon using tributyl phosphate. J Biomed Mater Res B. 2011;96:199–206.
Deeken CR, Fox DB, Bachman SL, Ramshaw BJ, Grant SA. Characterization of bionanocomposite scaffolds comprised of amine-functionalized gold nanoparticles and silicon carbide nanowires crosslinked to an acellular porcine tendon. J Biomed Mater Res B. 2011;97:334–44.
Deeken CR, Cozad MJ, Bachman SL, Ramshaw BJ, Grant SA. Characterization of bionanocomposite scaffolds comprised of amine-functionalized single-walled carbon nanotubes crosslinked to an acellular porcine tendon. J Biomed Mater Res A. 2011;96:584–94.
Deeken CR, Esebua M, Bachman SL, Ramshaw BJ, Grant SA. Assessment of the biocompatibility of two novel, bionanocomposite scaffolds in a rodent model. J Biomed Mater Res B. 2011;96:351–9.
Grant SA, Deeken CR, Grant DA, Bachman SL, Ramshaw BJ. In vivo study of a novel AuNP-tissue scaffold. Orlando: Society for Biomaterials; 2011.
Bellino MG, Calvo EJ, Gordillo G. Adsorption kinetics of charged thiols on gold nanoparticles. Phys Chem Chem Phys. 2004;6:424–8.
Cartmell JS, Dunn MG. Effect of chemical treatments on tendon cellularity and mechanical properties. J Biomed Mater Res. 2000;49:134–40.
Duan X, Sheardown H. Crosslinking of collagen with dendrimers. J Biomed Mater Res A. 2005;75:510–8.
Friess W, Lee G. Basic thermoanalytical studies of insoluble collagen matrices. Biomaterials. 1996;17:2289–94.
Ozaki Y, Mizuno A, Kaneuchi F. Structural differences between type-I and type-IV collagen in biological tissues studied invivo by attenuated total reflection Fourier-transform infrared-spectroscopy. Appl Spectrosc. 1992;46:626–30.
Deeken CR, Eliason BJ, Pichert MD, Grant SA, Frisella MM, Matthews BD: Characterization of the physicomechanical, thermal, and degradation properties of biologic scaffold materials utilized for hernia repair applications. In: Hernia Repair 2011, American Hernia Society, San Francisco, CA; 2011.
Rochdi A, Foucat L, Renou JP. Effect of thermal denaturation on water–collagen interactions: NMR relaxation and differential scanning calorimetry analysis. Biopolymers. 1999;50:690–6.
Than P, Halmai V, Kereskai L, Gazso I. Thermal analysis of the cruciate ligaments of the human knee. J Therm Anal Calorim. 2005;81:307–10.
Zeeman R, Dijkstra PJ, van Wachem PB, van Luyn MJ, Hendriks M, Cahalan PT, Feijen J. Successive epoxy and carbodiimide cross-linking of dermal sheep collagen. Biomaterials. 1999;20:921–31.
Olde Damink LH, Dijkstra PJ, van Luyn MJ, van Wachem PB, Nieuwenhuis P, Feijen J. In vitro degradation of dermal sheep collagen cross-linked using a water-soluble carbodiimide. Biomaterials. 1996;17:679–84.
Zerris VA, James KS, Roberts JB, Bell E, Heilman CB. Repair of the dura mater with processed collagen devices. J Biomed Mater Res B. 2007;83:580–8.
Liang HC, Chang Y, Hsu CK, Lee MH, Sung HW. Effects of crosslinking degree of an acellular biological tissue on its tissue regeneration pattern. Biomaterials. 2004;25:3541–52.
Wang XH, Li DP, Wang WJ, Feng QL, Cui FZ, Xu YX, Song XH, van der Werf M. Crosslinked collagen/chitosan matrix for artificial livers. Biomaterials. 2003;24:3213–20.
Acknowledgments
The scanning electron microscope utilized in this study was an FEI Quanta FEG 600 FEG acquired under NSF award #ECS-0619607, award #PRM-06-029, and other internal funds from the University of Missouri. This research was supported in part by a National Science Foundation Graduate Research Fellowship, an Internal Project Award from the Department of Surgery at the University of Missouri, and the University of Missouri Food for the twenty-first century (F21C) grant. The authors would also like to thank David Grant, J. Todd Vassalli, and Braden Eliason for their contributions to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deeken, C.R., Bachman, S.L., Ramshaw, B.J. et al. Characterization of bionanocomposite scaffolds comprised of mercaptoethylamine-functionalized gold nanoparticles crosslinked to acellular porcine tissue. J Mater Sci: Mater Med 23, 537–546 (2012). https://doi.org/10.1007/s10856-011-4486-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-011-4486-1