Abstract
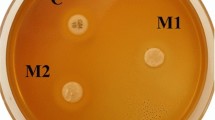
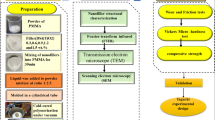
Inorganic-polymer nanocomposites are of significant interest for emerging materials due to their improved properties and unique combination of properties. Poly (methylmethacrylate) (PMMA)/montmorillonite (MMT) nanocomposites were prepared by in situ suspension polymerization with dodecylamine used as MMT-modifier. X-ray diffraction (XRD) and transmission electron microscopy (TEM) were used to characterize the structures of the nanocomposites. Cytotoxicity test, hemolysis test, acute systemic toxicity test, oral mucous membrane irritation test, guinea-pig maximization test and mouse bone-marrow micronucleus test were used to evaluate the biocompatibility of PMMA/MMT nanocomposites. The results indicated that an exfoliated nanocomposite was achieved, and the resulting nanocomposites exhibited excellent biocompatibility as denture base material and had potential application in dental materials.





Similar content being viewed by others
References
Kojima Y, Usuki A, Kawasumi M, Okada A, Kurauchi T, Kamigaito O. Synthesis of nylon 6-clay hybrid by montmorillonite intercalated with ε-caprolactam. J Polym Sci Part A Polym Chem. 1993;31:983–6.
Vaia RA, Vasudevan S, Krawiec W, Scanlon LG, Giannelis EP. New polymer electrolyte nanocomposites: melt intercalation of poly (ethylene oxide) in mica-type silicates. Adv Mater. 1995;7:154–6.
Kawasumi M, Hasegawa N, Kato M, Okada A. Preparation and mechanical-properties of polypropylene-clay hybrids. Macromolecules. 1997;30:6333–8.
Khaled SM, Sui RH, Charpentier PA, Rizkalla AS. Synthesis of TiO2-PMMA nanocomposite: using methacrylic acid as a coupling agent. Langmuir. 2007;23:3988–95.
Shen Z, Simon GP, Cheng Y. Nanocomposites of poly(methyl methacrylate) and organically modified layered silicates by melt intercalation. J Appl Polym Sci. 2004;92:2101–15.
Chen GH, Chen XQ, Lin ZY, Ye W, Yao KD. Preparation and properties of PMMA/clay nanocomposite. J Mater Sci Lett. 1999;18:1761–3.
Lee DC, Jang LW. Preparation and characterization of PMMA-clay hybrid composite by emulsion polymerization. J Appl Polym Sci. 1996;61:1117–22.
Salahuddin N, Shehata M. Polymethylmethacrylate-montmorillonite composites: preparation, characterization and properties. Polymer. 2001;42:8379–85.
Moursi AM, Winnard AV, Winnard PL, Lannutti JJ, Seghi RR. Enhanced osteoblast response to a polymethylmethacrylate–hydroxyapatite composite. Biomaterials. 2002;23:133–44.
Langer K, Marburger C, Berthold A, Kreuter J, Stieneker F. Methylmethacrylate sulfopropylmethacrylate copolymer nanoparticles for drug delivery part I: preparation and physicochemical characterization. Int J Pharm. 1996;137:67–74.
Huang X, Brittain WJ. Synthesis and characterization of PMMA nanocomposites by suspension and emulsion polymerization. Macromolecules. 2001;34:3255–60.
Kim SS, Park TS, Shin BC, Kim YB. Polymethyl methacrylate/montmorillonite nanocomposite beads through a suspension polymerization-derived process. J Appl Polym Sci. 2005;97:2340–9.
Mohammad A, Laleh S, Azizollah N, Seyed MM, Shahin K, Khatereh A, Samal B. PMMA-grafted nanoclay as novel filler for dental adhesives. Dent Mater. 2009;25:339–47.
Discacciati JAC, Oréfice RL. Structural analysis on photopolymerized dental resins containing nanocomponents. J Mater Sci. 2007;42:3883–93.
Kaaber S, Thulin H, Nielsen E. Skin sensitivity to denture base materials in the burning mouth syndrome. Contact Dermat. 1979;5:90–6.
Ruyter IE. Release of formaldehyde from denture base polymers. Acta Odontol Scand. 1980;38:17–27.
Moharamzadeh K, Brook IM, Van Noort R, Scutt AM. HPLC analysis of components released from dental composites with different resin compositions using different extraction media. J Mater Sci Mater Med. 2007;18:133–7.
Kedjarune U, Charoenworaluk N, Koontongkaew S. Release of methyl methacrylate from heat-cured and autopolymerized resins: cytotoxicity testing related to residual monomer. Aust Dent J. 1999;44:25–30.
Tuncel A, Ozdemir AK, Sumer Z, Hurmuzlu F, Polat Z. Cytotoxicity evaluation of two different composites with/without fibers and one nanohybrid composite. Dent Mater J. 2006;25:267–71.
Moharamzadeh K, Brook IM, Van Noort R, Scutt AM, Smith KG, Thornhill MH. Development, optimization and characterization of a full-thickness tissue engineered human oral mucosal model for biological assessment of dental biomaterials. J Mater Sci Mater Med. 2008;19:1793–801.
Jorge JH, Giampaolo ET, Machado AL, Vergani CE. Cytotoxicity of denture base acrylic resins: a literature review. J Prosthet Dent. 2003;90:190–3.
Van Kooten TG, Klein CL, Kohler H, Kirkpatrick CJ. From cytotoxicity to biocompatibility testing in vitro: cell adhesion molecule expression defines a new set of parameters. J Mater Sci Mater Med. 1997;8:835–41.
ISO 10993-1:2003 Biological evaluation of medical devices-part 1: evaluation and testing. Geneva: International Organization for Standardization; 2003. p. 1–10.
ISO 7405-1997 Dentistry-preclinical evaluation of biocompatibility of medical devices used in dentistry-test methods for dental materials. Geneva: International Organization for Standardization; 1997. p. 1–19.
American National Standard/American Dental Association specification No. 96, Dental water-based cements.
Hanks CT, Wataha JC, Sun Z. In vitro models of biocompatibility: a review. Dent Mater. 1996;12:186–93.
ISO 10993-5:1992 Biological evaluation of medical devices-part 5: tests for cytotoxicity: in vitro methods. Geneva: International Organization for Standardization; 1992. p. 1–8.
ISO 6344-1. Coated abrasives—grain size analysis. Geneva: International Organization for Standardization; 1998. p. 1–6.
Edmondson JM, Armstrong LS, Martinez AO. A rapid and simple MTT-based spectrophotometric assay for determining drug sensitivity in monolayer cultures. J Tissue Cult Meth. 1988;11:15–7.
ISO/TR 7405-1984(E) Heamolysis test (in vitro test directly on materials).
Zhang L, Jin G. Novel method for bilirubin removal from human plasma within modified polytetrafluoroethylene capillary. React Funct Polym. 2006;66:1106–17.
Delaney B, Shen ZR, Powley CR, Gannon S, Munley SA, Maxwell C, Barnett JF. Acute and repeated dose oral toxicity of N-acetyl-l-aspartic acid in Sprague–Dawley rats. Food Chem Toxicol. 2008;46:2023–34.
Botham PA. Acute systemic toxicity—prospects for tiered testing strategies. Toxicol In Vitro. 2004;18:227–30.
IS0 10993-2: 1992, Biological evaluation of medical devices-part 2: animal welfare requirements. Geneva: International Organization for Standardization; 1992. p. 1–3.
ISO 10993-10 Biological evaluation of medical devices-part 10: tests for irritation and delayed-type hypersensitivity. Geneva: International Organization for Standardization; 2002. p. 1–44.
Isbrucker RA, Edwards JA, Wolz E, Davidovich A, Bausch J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: dermal, acute and short-term toxicity studies. Food Chem Toxicol. 2006;44:636–50.
Kreiling R, Hollnagel HM, Hareng L, Eigler D, Lee MS, Griem P, Dreeßen B, Kleber M, Albrecht A, Garcia C, Wendel A. Comparison of the skin sensitizing potential of unsaturated compounds as assessed by the murine local lymph node assay (LLNA) and the guinea pig maximization test (GPMT). Food Chem Toxicol. 2008;46:1896–904.
Yamano T, Shimizu M, Noda T. Quantitative comparison of the results obtained by the multiple-dose guinea pig maximization test and the non-radioactive murine local lymph-node assay for various biocides. Toxicology. 2005;211:165–75.
Suter W, Martus HJ, Elhajouji A. Methylphenidate is not clastogenic in cultured human lymphocytes and in the mouse bone-marrow micronucleus test. Mutat Res. 2006;607:153–9.
MacGregor JT, Heddle JA, Hite M, Margolin BH. Guidelines for the conduct of micronucleus assays in mammalian bone marrow erythrocytes. Mutat Res. 1987;189:103–12.
Wang HF, Wang J, Deng XY. Preparation and biological behaviors of 1251-labeled water-soluble single-wall carbon nanotubes. Nanosci Nanotechnol. 2004;4:1–6.
Zhang C, Qu G, Sun Y, Wu X, Yao Z, Guo Q, Ding Q, Yuan S, Shen Z, Ping Q, Zhou H. Pharmacokinetics, biodistribution, efficacy and safety of N-octyl-O-sulfate chitosan micelles loaded with paclitaxel. Biomaterials. 2008;29:1233–41.
Acknowledgments
The authors are grateful for the extensive laboratory assistance of Microbiology Laboratory of Basic Medical College and Toxicology Laboratory of Public Health College, Tianjin Medical University. This investigation was supported by Program for New Century Excellent Talents in University, People’s Republic of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, J., Su, Q., Wang, C. et al. Synthesis and biological evaluation of PMMA/MMT nanocomposite as denture base material. J Mater Sci: Mater Med 22, 1063–1071 (2011). https://doi.org/10.1007/s10856-011-4269-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-011-4269-8