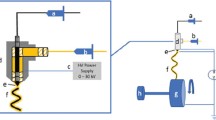
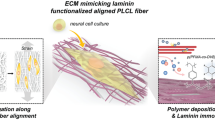
Abstract
Microfibers produced with electrospinning have recently been used in tissue engineering. In the development of artificial implants for nerve regeneration they are of particular interest as guidance structures for cell migration and axonal growth. Using electrospinning we produced parallel-orientated biocompatible fibers in the submicron range consisting of poly(ε-caprolactone) (PCL) and star shaped NCO-poly(ethylene glycol)-stat-poly(propylene glycol) (sPEG). Addition of the bioactive peptide sequence glycine-arginine-glycine-aspartate-serine (GRGDS) or the extracellular matrix protein fibronectin to the electrospinning solution resulted in functionalized fibers. Surface characteristics and biological properties of functionalized and non-functionalised fibers were investigated. Polymer solutions and electrospinning process parameters were varied to obtain high quality orientated fibers. A polymer mixture containing high molecular weight PCL, PCL-diol, and sPEG permitted a chemical reaction between hydroxyl groups of the diol and isocyanante groups of the sPEG. Surface analysis demonstrated that sPEG at the fiber surface minimized protein adhesion. In vitro experiments using dorsal root ganglia explants showed that the cell repellent property of pure PCL/sPEG fibers was overcome by functionalization either with GRGDS peptide or fibronectin. In this way cell migration and axonal outgrowth along fibers were significantly increased. Thus, functionalized electrospun PCL/sPEG fibers, while preventing non-specific protein adsorption, are a suitable substrate for biological and medical applications.









Similar content being viewed by others
Abbreviations
- BSA:
-
Bovine serum albumin
- DAPI:
-
4′,6-Diamidino-2-phenylindole dihydrochloride
- DCM:
-
Dichloromethane
- DIV:
-
Days in vitro
- DMEM:
-
Dulbecco’s modified eagle medium
- DMSO:
-
Dimethyl sulfoxide
- DRG:
-
Dorsal root ganglia
- ECM:
-
Extracellular matrix
- FCS:
-
Fetal calf serum
- GRGDS:
-
One letter code of the peptide sequence GlyArgGlyAspSer
- Mw :
-
Molecular weight
- NF200:
-
Neurofilament 200 kDa
- NGS:
-
Normal goat serum
- NMP:
-
N-Methylpyrrilidone
- PBS:
-
Phosphate buffer saline
- PCL:
-
Poly(ε-caprolactone)
- PCL-ol:
-
PCL diol
- PEG:
-
Poly(ethylene glycol)
- PNS:
-
Peripheral nervous system
- S100:
-
Protein antibody, marker for Schwann cells
- SC:
-
Schwann cells
- SEM:
-
Scanning electron microscopy
- sPEG:
-
Star shaped NCO-poly(ethylene glycol)-stat-poly(propylene glycol)
- THF:
-
Tetrahydrofuran
- TBS-T:
-
Tris-buffered saline with 1% Triton-X 100
- wt%:
-
Weight percent
- XPS:
-
X-ray photoelectron spectroscopy
References
Lietz M, Dreesmann L, Hoss M, Oberhoffner S, Schlosshauer B. Neuro tissue engineering of glial nerve guides and the impact of different cell types. Biomaterials. 2006;27:1425–36.
Schnell E, Klinkhammer K, Balzer S, Brook G, Klee D, Dalton P, Mey J. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-ε-caprolactone and a collagen/poly-ε-caprolactone blend. Biomaterials. 2007;28(19):3012–25.
Smeal RM, Rabbitt R, Biran R, Tresco PA. Substrate curvature influences the direction of nerve outgrowth. Ann Biomed Eng. 2005;33(3):376–82.
Li D, Xia YN. Electrospinning of nanofibers: reinventing the wheel? Adv Mater. 2004;16(14):1151–70.
Huang ZM, Zhang YZ, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol. 2003;63(15):2223–53.
Klinkhammer K, Seiler N, Grafahrend D, Gerardo-Nava J, Mey J, Brook GA, Möller M, Dalton PD, Klee D. Deposition of electrospun fibers on reactive substrates for in vitro investigations. Tissue Eng. 2009;15(1):77–85.
Horbett TA. The role of adsorbed proteins in tissue response to biomaterials. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterials science: an introduction to materials in medicine. San Diego: Elsevier; 2004. p. 237–40.
Tang L, Jennings TA, Eaton JW. Mast cells mediate acute inflammatory responses to implanted biomaterials. PNAS. 1998;95:8841–6.
Johnson R, Harrison D, Tucci M, Tsao A, Lemos M, Puckett A, Hughes JL, Benghuzzi H. Fibrous capsule formation in response to ultrahigh molecular weight polyethylene treated with peptides that influence adhesion. Biomed Sci Instrum. 1997;34:47–52.
Jeon SI, Lee JH, Andrade JD, De Gennes PG. Protein-surface interactions in the presence of polyethylene oxide: I. Simplified theory. J Colloid Interface Sci. 1991;142:149–58.
Sharma S, Johnson RW, Desai TA. Evaluation of the stability of nonfouling ultrathin poly(ethylene glycol) films for silicon-based microdevices. Langmuir. 2004;20(2):348–56.
Grafahrend D, Calvet JL, Klinkhammer K, Salber J, Dalton PD, Moller M, Klee D. Control of protein adsorption on functionalized electrospun fibers. Biotechnol Bioeng. 2008;101(3):609–21.
Gasteier P, Reska A, Schulte P, Salber J, Offenhausser A, Moeller M, Groll J. Surface grafting of PEO-based star-shaped molecules for bioanalytical and biomedical applications. Macromol Biosci. 2007;7(8):1010–23.
Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24:4385–415.
Goetz H, Beginn U, Bartelink CF, Grunbauer HJM, Möller M. Preparation of isophorone diisocyanate terminated star polyethers. Macromol Mater Eng. 2002;287(4):223–30.
Grafahrend D, Calvet JL, Salber J, Dalton PD, Moeller M, Klee D. Biofunctionalized poly(ethylene glycol)-block-poly(epsilon-caprolactone) nanofibers for tissue engineering. J Mater Sci Mater Med. 2008;19(4):1479–84.
Groll J, Fiedler J, Engelhard E, Ameringer T, Tugulu S, Klok HA, Brenner RE, Moeller M. A novel star PEG-derived surface coating for specific cell adhesion. J Biomed Mater Res Part A. 2005;74A(4):607–17.
Grafahrend D, Gasteier P, Heffels K-H, Beer M, Dalton PD, Moeller M, Groll J. Transforming the surface chemistry of degradable polymer scaffolds with a reactive hydrophilic monomer additive. Submitted.
Hsu C-M, Shivkumar S. Nano-sized beads and porous fiber constructs of poly(ε-caprolactone) produced by electrospinning. J Mater Sci. 2004;39(9):3003–13.
Doshi J, Reneker DH. Electrospinning process and applications of electrospun fibers. J Electrost. 1995;35(2–3):151–60.
Liu HQ, Hsieh YL. Ultrafine fibrous cellulose membranes from electrospinning of cellulose acetate. J Polym Sci Part B Polym Phys. 2002;40:2119–29.
Andrady AL. Science and technology of polymer nanofibers. Hoboken: Wiley; 2008. p. 94.
Bölgen N, Menceloglu YZ, Acatay K, Vargel I, Piskin E. In vitro and in vivo degradation of non-woven materials made of poly(ε-caprolactone) and nanofibers prepared by electrospinning under different conditions. J Biomater Sci Polym Ed. 2005;16(12):1537–55.
Park JB, Yiu G, Kaneko S, Wang J, Chang J, He Z. A TNF receptor family member, TROY, is a coreceptor with Nogo receptor in mediating the inhibitory activity of myelin inhibitors. Neuron. 2005;45:345–51.
Vyas AA, Blixt O, Paulson JC, Schnaar RL. Potent glycan inhibitors of myelin-associated glycoprotein enhance axon outgrowth in vitro. J Biol Chem. 2005;280(16):16305–10.
Filbin MT, Qiu J, Cai D. Glial influences on axonal regeneration. In: Jessen KR, Richardson WD, editors. Glial cell development-basic principles and clinical relevance. Oxford: Oxford University Press; 2001. p. 279–98.
Lisak RP, Skundric D, Bealmear B, Ragheb S. The role of cytokines in Schwann cell damage, protection, and repair. J Infect Dis. 1997;176:S173–9.
Schense JC, Bloch J, Aebischer P, Hubbell JA. Enzymatic incorporation of bioactive peptides into fibrin matrices enhances neurite extension. Nat Biotechnol. 2000;18:415–9.
Tong YW, Shoichet MS. Enhancing the neuronal interaction on fluoropolymer surfaces with mixed peptides or spacer group linkers. Biomaterials. 2001;22:1029–34.
Patel S, Kurpinski K, Quigley R, Gao H, Hsiao BS, Poo M-M, Li S. Bioactive nanofibers: synergistic effects of nanotopography and chemical signaling on cell guidance. Nano Lett. 2007;7(7):2122–8.
Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3.
Akiyama SK, LaFlamme SE. Bioadhesion and cell behavior. Colloids Surf B Biointerfaces. 1994;2:241–50.
Zhang Z, Yoo R, Wells M, Beebe TP Jr, Biran R, Tresco P. Neurite outgrowth on well-characterized surfaces: preparation and characterization of chemically and spatially controlled fibronectin and RGD substrates with good bioactivity. Biomaterials. 2005;26:47–61.
Schlosshauer B, Dreesmann L, Schaller H-E, Sinis N. Synthetic nerve guide implants in humans: a comprehensive survey. Neurosurgery. 2006;59:470–8.
Möllers S, Heschel I, Damink LH, Schugner F, Deumens R, Muller B, Bozkurt A, Nava JG, Noth J, Brook GA. Cytocompatibility of a novel, longitudinally microstructured collagen scaffold intended for the nerve tissue repair. Tissue Eng Part A. 2009;15:461–72.
Yu TT, Shoicht MS. Guided cell adhesion and outgrowth in peptide-modified channels for tissue engineering. Biomaterials. 2005;26:1507–14.
Kim YT, Haftel VK, Kumar S, Bellamkonda RV. The role of aligned polymer fiber-based constructs in the bridging of long peripheral nerve gaps. Biomaterials. 2008;29:3117–27.
Lundborg G, Dahlin L, Dohi D, Kanje M, Terada N. A new type of “bioartificial” nerve graft for bridging extended defects in nerves. J Hand Surg (British and European Volume). 1997;22B:299–303.
Matsumoto K, Ohnishi K, Kiyotani T, Sekine T, Ueda H, Nakamura T, Endo Y, Shimizu Y. Peripheral nerve regeneration across an 80-mm gap bridged by a polyglycolic acid (PGA)-collagen tube filled with laminin-coated collagen fibers: a histological and electrophysical evaluation of regenerated nerves. Brain Res. 2000;868:315–28.
Acknowledgments
We thank Marie Pradella for help with evaluating the cell experiments. Kristin Michael and Bernd Hoffmann kindly allowed us to use their equipment for making composite photographs of DRG explants. This work was supported by a grant from the Interdisciplinary Centre for Clinical Research “BIOMAT” within the faculty of Medicine at Aachen University (RWTH) (TV B111) and by DFG-Graduiertenkolleg 1035 “Biointerface”. Julia Bockelmann was supported by a Marie-Curie EST grant from the EU (EURON).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klinkhammer, K., Bockelmann, J., Simitzis, C. et al. Functionalization of electrospun fibers of poly(ε-caprolactone) with star shaped NCO-poly(ethylene glycol)-stat-poly(propylene glycol) for neuronal cell guidance. J Mater Sci: Mater Med 21, 2637–2651 (2010). https://doi.org/10.1007/s10856-010-4112-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-010-4112-7