Abstract
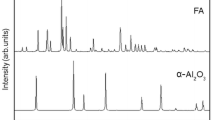
Silicon (Si) substitution in the crystal structure of calcium phosphate (CaP) ceramics has proved to generate materials with improved bioactivity than their stoichiometric counterpart. In light of this, in the current work, 100 wt% hydroxyapatite (HA) precursor and 25 wt% SiO2-HA precursors were used to prepare bioactive coatings on Ti-6Al-4V substrates by a laser cladding technique. The effects of SiO2 on phase constituents, crystallite size, surface roughness, and surface energy of the CaP coatings were studied. Furthermore, on the basis of these results, the effects and roles of SiO2 substitution in HA were systematically discussed. X-ray diffraction analysis of the coated samples indicated the presence of various phases such as CaTiO3, Ca2SiO4, Ca3(PO4)2, TiO2 (Anatase), and TiO2 (Rutile). The addition of SiO2 in the HA precursor resulted in the refinement of grain size. Confocal laser microscopy characterization of the surface morphology demonstrated an improved surface roughness for samples with 25 wt% SiO2-HA precursor compared to the samples with 100 wt% HA precursor processed at 125 cm/min laser speed. The addition of SiO2 in the HA precursor resulted in the highest surface energy, increased hydrophilicity, and improved biomineralization as compared to the control (untreated Ti-6Al-4V) and the sample with 100 wt% HA as precursor. The microstructural evolution observed using a scanning electron microscopy indicated that the addition of SiO2 in the HA precursor resulted in the presence of reduced cracking across the cross-section of the bioceramic coating.






Similar content being viewed by others
References
Driessens FCM, Verbeeck RMH. Biomaterials. Boca Raton: CRC Press; 1990. p. 5.
Foppiano S, Marshall SJ, Marshall GW, Saiz E, Tomsia AP. The influence of novel bioactive glasses on in vitro osteoblast behavior. J Biomed Mater Res. 2004;71:242–9.
Bosetti M, Cannas M. The effect of bioactive glasses on bone marrow stromal cells differentiation. Biomaterials. 2005;26:3873–9.
Hattar S, Asselin A, Greenspan D, Oboeuf M, Berdal A, Sautier JM. Potential of biomimetic surfaces to promote in vitro osteoblast-like cell differentiation. Biomaterials. 2005;26:839–48.
Lopez-Heredia MA, Legeay G, Gaillard C, Layrolle P. Radio frequency plasma treatments on titanium for enhancement of bioactivity. Acta Biomater. 2008;4:1953–62.
Pecheva Emilia V, Pramatarova Liliana D, Maitz Manfrfed F, Pham Mihn T, Kondyurin Aleye V. Kinetics of hydroxyapatite deposition on solid substrates modified by sequential implantation of Ca and P ions: Part I. FTIR and Raman spectroscopy study. Appl Surf Sci. 2004;235:176–81.
Weng J, Liu Q, Wolke JGC, Zhang XD, de Groot K. Formation and characteristics of the apatite layer on plama-sprayed hydroxyapatite coatings in simulated body fluid. Biomaterials. 1997;18:1027–35.
Bang HG, Kim SJ, Park SY. Biocompatibility and the physical properties of bio-glass ceramics in the Na2O-CaO-SiO2-P2O5 system with CaF2 and MgF2 additives. J Ceram Process Res. 2008;9:588–90.
Hristov JH, Bogdanov BI, Chomakov IG, Markov IG, Markovska IG. Drawing standard curve for quantitative determination of the crystalline phase in wollastonite glass ceramics. J Balkan Tribolog Assoc. 2009;15:347–54.
Choi SW, Hong SH, Kim YJ. Characterization of Ca2SiO4: Eu2+ phosphors synthesized by polymeric precursor process. J Am Ceram Soc. 2009;92:2025–8.
Carlise E. Si: an essential element for the chick. Science. 1972;178:619–21.
Schwarz K, Milne D. Growth promoting effects of Si in rats. Nature. 1972;239:333–4.
Seaborn C, Nielson F. Si depravation decreases collagen formation in wounds, bone and ornithine transaminase enzyme activity in liver. Biol Trace Elem Res. 2002;89:251–61.
Schwarz K. A bound form of Si in glycosaminoglycans and polyuronides. Proc Nat Acad Sci USA. 1973;70:1608–12.
Pietak AM, Reid JW, Stott MJ, Sayer M. Silicon substitution in the calcium phosphate bioceramics. Biomaterials. 2007;28:4023–32.
Tanizawa Y, Suzuki T. Effects of silicate ions on the formation and transformation of calcium phosphates in neutral aqueous solutions. J Chem Soc Faraday Trans. 1995;91:3499–503.
Damen J, Ten Cate J. Silica-induced precipitation of calcium phosphate in the presence of inhibitors of hydroxyapatite formation. J Dent Res. 1992;71:453–7.
Sayer M, Stratilatov A, Reid J, Calderin L, Stott M, Yin X, et al. Structure and composition of silicon stabilized tricalcium phosphate. Biomaterials. 2002;24:369–82.
Gibson I, Best S, Bonfield W. Effect of silicon substitution on the sintering and microstructure of hydroxyapatite. J Am Ceram Soc. 2002;85:2771–7.
Tang X, Xiao X, Liu R. Structural characterization of silicon substituted hydroxyapatite synthesized by hydrothermal method. Mater Lett. 2005;59:3841–6.
Reid J, Tuck L, Sayer M, Fargo K, Hendry J. Synthesis and characterization of single-phase silicon-substituted alpha-tricalcium phosphate. Biomaterials. 2006;27:2915–6.
Cheng XM, Nie BM, Kumar S. Preparation and bioactivity of SiO2 functional films on titanium by PACVD. Trans Nonferrous Met Soc China. 2008;18:627–30.
Acros D, Rodriguez-Carvajal J, Vallet-Regi M. Silicon incorporation in hydroxyapatite obtained by controlled crystallization. Chem Mater. 2004;16:2300–8.
Pietak A, Sayer M. Crystallization kinetics of Si-TCP bioceramic. J Mater Sci. 2004;39:2443–9.
Patel N, Best S, Bonfield W, Gibson I, Hing K, Damien E, et al. A comparative study on the in vivo behavior of hydroxyapatite and silicon substituted hydroxyapatite granules. J Mater Sci Mater Med. 2002;13:1199–206.
Thian ES, Ahmad Z, Huang J, Edirisinghe MJ, Jayasinghe SN, Ireland DC, Brooks RA, Rushton N, Bonfield W, Best SM. The role of surface wettability and surface charge of electrosprayed nanoapatites on the behavior of osteoblasts. Acta Biomater. 2010;6:750–5.
Cullity BD. Elements of X-ray diffraction. 2nd ed. Reading: Addison-Wesley Publishing Company, Inc; 1978. p. 284.
Van Oss CJ, Good RJ, Chaudhury MK. Additive and non additive surface tension components and the interpretation of contact angles. Langmuir. 1988;4:884–91.
Randeniya LK, Bendavid A, Martin PJ, Amin MS, Preston EW, Ismail FSM, Coe S. Incorporation of Si and SiOx into diamond-like carbon films: impact on surface properties and osteoblast adhesion. Acta Biomater. 2009;5:1791–7.
Van Oss CJ, Giese RF Jr, Good RJ. Reevaluation of the surface tension components and parameters of polyacetylene from contact angle of liquids. Langmuir. 1990;6:1711–3.
Roseman RD, Mukherjee N. PTCR effect in BaTiO3: structural aspects and grain boundary potentials. J Electroceram. 2003;10:117–35.
Zubair MA, Leach C. The effect of SiO2 addition on the development of low-∑ grain boundaries in PTC thermistors. J Eur Ceram Soc (in press, available on line).
Guan K, Lu B, Yin Y. Enhanced effect and mechanism of SiO2 addition in super-hydrophilic property of TiO2 films. Surf Coat Technol. 2003;173:219–23.
Tekeli S, Erdogan M, Aktas B. Structural evolution in 8 mol% Y2O3-stabilized cubic zirconia (8YSCZ) with SiO2 addition. Mater Sci Eng A. 2004;386:1–9.
Tekeli S, Boyacioğlu T, Gűral A. The effect of silica doping on the microstructure and mechanical properties of c-ZrO2/SiO2 composites. Ceram Int. 2008;34:1959–64.
Brook RJ. Treatise on materials science and technology. New York: Academic Press; 1976.
Yoo Y-S, Kim H, Kim D-Y. Effect of SiO2 and TiO2 addition on the exaggerated grain growth of BaTiO3. J Eur Ceram Soc. 1997;17:805–11.
Hussaina S, Anis-ur-Rehmanb M, Maqsooda A, Awan MS. The effect of SiO2 addition on structural, magnetic and electrical properties of strontium hexa-ferrites. J Cryst Growth. 2006;297:403–10.
Paital SR, Dahotre NB. A thermal model for laser interaction with thick dielectric film on metallic substrate: application to Ca-P layer on Ti alloy. J Alloys Compd. 2009;487:499–503.
Eustathopoulos N, Nicholas MG, Drevet B. Wettability at high temperatures. 2nd ed. New York: Pergamon; 1999. p. 108.
Ishida K. Effect of grain size on grain boundary segregation. J Alloys Compd. 1996;235:244–9.
Izquierdo-Barba I, Conde F, Olmo N, Lizarbe MA. Vitreous SiO2-CaO coatings on Ti6Al4V alloys: reactivity in simulated body fluid versus osteoblast cell culture. Acta Biomater. 2006;2:445–55.
Ducheyne P, Qui Q. Bioactive ceramics: the effect of surface reactivity on bone formation and function. Biomaterials. 1999;20:2287–303.
Hench L. Surface reaction kinetics and adsorption of biological moieties: a mechanistic approach to tissue attachment. In: Davies JE, editor. The bone biomaterial interface. Toronto: University of Toronto Press; 1991. p. 33–42.
Acknowledgments
A support to Yuling Yang during this work at the University of Tennessee by National Science Foundation of China for Young Scholars (Grant # 50801012) is highly acknowledged. Yuling Yang also thanks China Scholarship Council (CSC) and Northeastern University (NEU) for providing a financial support as visiting scholar at the University of Tennessee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Y., Paital, S.R. & Dahotre, N.B. Effects of SiO2 substitution on wettability of laser deposited Ca-P biocoating on Ti-6Al-4V. J Mater Sci: Mater Med 21, 2511–2521 (2010). https://doi.org/10.1007/s10856-010-4105-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-010-4105-6