Abstract
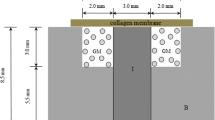
Background Reconstruction of large segment of bony defects is frequently needed in hand surgery. Autogenous bone grafting is considered the standard in management of these bony defects but has limited source of graft material. Collagen and hydroxyapatite have been used as bone-filling materials and are known to serve as the osteoconductive scaffold for bone regeneration. On the other hand, platelet-rich plasma is a kind of natural source of growth factors, and has been used successfully in bone regeneration and improving wound healing. This study was designed to evaluate the effectiveness of using biohybrids of platelet-rich plasma and collagen-hydroxyapatite beads for fabricating of protrusive bone in a rabbit animal model. Methods Biomaterial beads comprised of particulate hydroxyapatite dispersed in fibrous collagen (type I) matrices were prepared and filled in the ringed polytetrafluoroethylene (PTFE) artificial vascular graft (3 cm long, 1 cm in diameter). New Zealand White rabbits were each implanted with two cylindrical PTFE artificial vascular graft over both iliac crests (n = 16). A 2 × 0.5 cm opening was created at the side of each PTFE chamber to allow the content of chamber in contact with the bone marrow of the ileum. The chambers were empty (groups A and D), filled with type I collagen/hydroxyapatite beads (groups B and C). In groups C and D, autologous platelet rich plasma (PRP) was given by transcutaneous injection method into the chambers every week. After 12 weeks, the animals were sacrificed and the chambers were harvested for radiological and histological analysis. Results In plain radiographs, the group C chambers had significantly higher bone tissue engineered (average calcified density 0.95, average calcified area 61.83%) compared with other groups (P < 0.001). In histological examination, there was a creeping substitution of the implant by the in-growth of fibrovascular tissue in group C. Abundant fibrovascular networks positioned interstitially between these biomaterial beads in all parts of chamber. Degradation of these beads and newly formed capillaries and osteoids around the degraded matrixes are shown. The continually calcification in the matrixes or degraded matrixes is evidenced by the strong green fluorescence observed under the confocal microscope. In group B, looser architecture without evidence of tissue in-growth was shown. In the scaffold absent groups (A and D), there was only fibrous tissue shown within the chamber. Conclusions In this study, we have demonstrated a feasible approach to fabricate an osseous tissue that meets clinical need. Using the type I collagen/ hydroxyapatite gel beads matrixes and intermittent injection of autologous platelet-rich-plasma, specific 3D osseous tissues with fibrovascular network structure from pre-exist bony margin were successfully created in an in vivo animal model.




Similar content being viewed by others
References
R. Langer, J.P. Vacanti, Tissue engineering. Science 260, 920–926 (1993). doi:10.1126/science.8493529
G.M. Crane, S.L. Ishaug, A.G. Mikos, Bone tissue engineering. Nat. Med. 1, 1322–1324 (1995). doi:10.1038/nm1295-1322
Y. Weng, Y. Cao, C.A. Silva, M.P. Vacanti, C.A. Vacanti, Tissue-engineered composites of bone and cartilage for mandible condylar reconstruction. J. Oral Maxillofac. Surg. 59, 185 (2001). doi:10.1053/joms.2001.20491
L.G. Cima, J.P. Vacanti, C. Vacanti, D. Ingber, D. Mooney, R. Langer, Tissue engineering by cell transplantation using degradable polymer substrates. J. Biomech. Eng. 113, 143–151 (1991). doi:10.1115/1.2891228
L.D. Shea, D. Wang, R.T. Franceschi, D.J. Mooney, Engineered bone development from a pre-osteoblast cell line on three-dimensional scaffolds. Tissue Eng. 6(6), 605–617 (2000). doi:10.1089/10763270050199550
M.C. Wake, C.W. Patrick, A.G. Mikos, Pore morphology effects on the fibrovascular tissue growth in porous polymer substrates. Cell Transplant. 3, 339–343 (1994)
T. Yoshikawa, H. Ohgushi, H. Nakajima et al., In vivo osteogenetic durability of cultured bone in porous ceramics: a novel method for autogenous bone graft substitution. Transplantation 69, 128 (2000). doi:10.1097/00007890-200001150-00022
N. Tamai, A. Myoui, T. Tomita et al., Novel hydroxyapatite ceramics with an interconnective porous structure exhibit superior osteoconduction in vivo. J. Biomed. Mater. Res. 59, 110 (2002). doi:10.1002/jbm.1222
H.A. Marouf, A.A. Quayle, P. Sloan, In vitro and in vivo studies with collagen/hydroxyapatite implants. Int. J. Oral Maxillofac. Implants 5, 148–154 (1990)
S. Kale, S. Biermann, C. Edwards, C. Tarnowski, M. Morris, M.W. Long, Three-dimensional cellular development is essential for ex vivo formation of human bone. Nat. Biotechnol. 18, 954 (2000). doi:10.1038/79439
A.K. Gosain, L. Song, P. Riordan et al., A 1-year study of osteoinduction in hydroxyapatite-derived biomaterials in an adult sheep model. Part I. Plast. Reconstr. Surg. 109, 619 (2002). doi:10.1097/00006534-200202000-00032
F.Y. Hsu, S.C. Chueh, Y.J. Wang, Microspheres of hydroxyapatite/reconstituted collagen as supports for osteoblast cell growth. Biomaterials 20, 1931–1936 (1999). doi:10.1016/S0142-9612(99)00095-2
H. Schilephake, Bone growth factors in maxillofacial skeletal reconstruction. Int. J. Oral Maxillofac. Surg. 31(5), 469–484 (2002). doi:10.1054/ijom.2002.0244
R.E. Marx, E.R. Carlson, R.M. Eichstaedt, S.R. Schimmele, J.E. Strauss, K.R. Georgeff, Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 85, 638–646 (1998). doi:10.1016/S1079-2104(98)90029-4
D. Sonnleitner, P. Huemer, D.Y. Sullivan, A simplified technique for producing platelet rich plasma and platelet concentrate for intraoral bone grafting techniques: a technical note. J. Oral Maxillofac. Implants 15, 879–882 (2000)
A. Dugrillon, H. Eichler, S. Kern, H. Kluter, Autologous concentrated platelet-rich plasma (cPRP) for local application in bone regeneration. Int. J. Oral Maxillofac. Surg. 31(6), 615–619 (2002). doi:10.1054/ijom.2002.0322
T. Oyama, S. Nishimoto, T. Tsugawa, F. Shimizu, Efficacy of platelet-rich plasma in alveolar bone grafting. J. Oral Maxillofac. Surg. 62(5), 555–558 (2004). doi:10.1016/j.joms.2003.08.023
J.J. de Obarrio, J.I. Arauz-Dutari, T.M. Chamberlain, A. Croston, The use of autologous growth factors in periodontal surgical therapy: platelet gel biotechnology—case reports. Int. J. Periodontics Restorative Dent. 20, 486–497 (2000)
C. Dahlin, A. Linde, J. Gottlow, S. Nyman, Healing of bone defects by guided tissue regeneration. Plast. Reconstr. Surg. 81, 672 (1988). doi:10.1097/00006534-198805000-00004
A. Hokugo, Y. Kubo, Y. Takahashi et al., Prefabrication of vascularized bone graft using guided bone regeneration. Tissue Eng. 10, 978 (2004)
I. Miyamoto, Y. Tsuboi, K. Takahashi et al., Enhancement of bone volume in guided bone augmentation by cell transplants derived from periosteum: an experimental study in rabbit calvarium bone. Clin. Oral Implants Res. 15, 308 (2004). doi:10.1111/j.1600-0501.2004.01011.x
R.C. Thomson, A.G. Mikos, E. Beahm, J.C. Lemon, W.C. Satterfield, T.B. Aufdemorte, M.J. Miller, Guided tissue fabrication from periosteum using preformed biodegradable polymer scaffolds. Biomaterials 20, 2007 (1999)
M. Kellomaki, H. Niiranen, K. Puumanen, N. Ashammakhi, T. Waris, P. Tormala, Bioabsorbable scaffolds for guided bone regeneration and generation. Biomaterials 21, 2495 (2000). doi:10.1016/S0142-9612(00)00117-4
L.L.H. Huang-Lee, M.E. Nimni, Preparation of type I collagen fibrillar matrices and the effects of collagen concentration of fibroblast contraction. Biomed. Eng. Appl. Basis Commun. 5, 664–675 (1993)
G. Merlino, M. Borsetti, M. Boltri, Reverse radial artery bone flap reconstruction of segmental metacarpal losses. J. Hand. Surg. [Br] 32(1), 98–101 (2007). doi:10.1016/j.jhsb.2006.08.015
H.-B. Lee, K.-C. Tark, S.-Y. Kang, S.-W. Kim, Y.-K. Chung, Reconstruction of composite metacarpal defects using a fibula free flap. Plast. Reconstr. Surg. 105(4), 1448–1452 (2000). doi:10.1097/00006534-200004040-00029
V.R. Hentz, J. Chang, Tissue engineering for reconstruction of the thumb. N. Engl. J. Med. 344, 1547–20 (2001). doi:10.1056/NEJM200105173442011
E. Sachlos, J.T. Czernuszka, Making tissue engineering scaffolds work. Review: the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur. Cell Mater. 5, 29–40 (2003)
D.-A. Wahl, E. Sachlos, C.-E. Liu, J.T. Czernuszka, Controlling the processing of collagen-hydroxyapatite scaffolds for bone tissue engineering. J. Mater. Sci. Mater. Med. 18(2), 201–209 (2007). doi:10.1007/s10856-006-0682-9
E. Sachlos, D. Gotora, J.T. Czernuszka, Collagen scaffolds reinforced with biomimetic composite nano-sized carbonate-substituted hydroxyapatite crystals and shaped by rapid prototyping to contain internal microchannels. Tissue Eng. 12(9), 2479–2487 (2006). doi:10.1089/ten.2006.12.2479
Acknowledgements
This work has been supported by grants from Ministry of Education-Aim for the Top University Plan, and from the Mackay Memorial Hospital, Taipei, Taiwan, Republic of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, SH., Hsu, YM., Wang, Y.J. et al. Fabrication of pre-determined shape of bone segment with collagen-hydroxyapatite scaffold and autogenous platelet-rich plasma. J Mater Sci: Mater Med 20, 23–31 (2009). https://doi.org/10.1007/s10856-008-3507-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-008-3507-1