Abstract
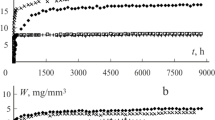
The water sorption and desorption behaviour of three commercial glass-ionomer cements used in clinical dentistry have been studied in detail. Cured specimens of each material were found to show slight but variable water uptake in high humidity conditions, but steady loss in desiccating ones. This water loss was found to follow Fick’s law for the first 4–5 h. Diffusion coefficients at 22 °C were: Chemflex 1.34 × 10−6 cm2 s−1, Fuji IX 5.87 × 10−7 cm2 s−1, Aquacem 3.08 × 10−6 cm2 s−1. At 7 °C they were: Chemflex 8.90 × 10−7 cm2 s−1, Fuji IX 5.04 × 10−7 cm2 s−1, Aquacem 2.88 × 10−6 cm2 s−1. Activation energies for water loss were determined from the Arrhenius equation and were found to be Chemflex 161.8 J mol−1, Fuji IX 101.3 J mol−1, Aquacem 47.1 J mol−1. Such low values show that water transport requires less energy in these cements than in resin-modified glass-ionomers. Fick’s law plots were found not to pass through the origin. This implies that, in each case, there is a small water loss that does not involve diffusion. This was concluded to be water at the surface of the specimens, and was termed “superficial water”. As such, it represents a fraction of the previously identified unbound (loose) water. Superficial water levels were: Chemflex 0.56%, Fuji IX 0.23%, Aquacem 0.87%. Equilibrium mass loss values were shown to be unaffected by temperature, and allowed ratios of bound:unbound water to be determined for all three cements. These showed wide variation, ranging from 1:5.26 for Chemflex to 1:1.25 for Fuji IX.






Similar content being viewed by others
References
A. D. WILSON and J. W. McLEAN, Glass Ionomer Cement (Quintessence Publishers, Chicago, 1988)
A. D. WILSON and J. W. NICHOLSON, Acid-Base Cements (The University Press, Cambridge 1994)
J. W. NICHOLSON, Biomaterials. 19 (1998) 485
A. D. WILSON, and S. CRISP, Br. Polym. J. 7 (1975) 279
H. J. PROSSER, and A. D. WILSON, J. Chem. Technol. Biotechnol. 29 (1979) 67
A. D. WILSON, S. CRISP, and J. M. PADDON, Br. Polym. J. 13 (1981) 66
J. A. BARRIE, in “Water in Polymers”, edited by J. CRANK and G.S. PARK (Academic Press, New York, 1969) pp. 259–313
M. BRADEN, B. E. CAUSTON and R. L. CLARKE, J. Dent. Res. 55 (1976) 730
S. KALACHANDRA and T. W. WILSON, Biomaterials 13 (1992) 105
M. BRADEN, and P. S. WRIGHT, J. Dent. Res. 62 (1983) 764
G. D. STAFFORD and M. BRADEN, J. Dent. Res. 47 (1968), 341
P. R. HORNSBY, J. Chem. Technol. Biotechnol. 30 (1980) 595
N. A. LANGE (edited by) in “Handbook of Chemistry” (McGraw-Hill, New York 1961) p. 1420
J. W. NICHOLSON, J. Mater. Sci. Mater. Med. 8 (1997) 691
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nicholson, J.W., Czarnecka, B. Kinetic studies of water uptake and loss in glass-ionomer cements. J Mater Sci: Mater Med 19, 1723–1727 (2008). https://doi.org/10.1007/s10856-007-3244-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-007-3244-x