Abstract
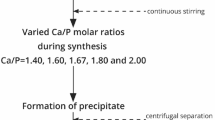
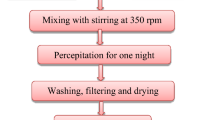
Fluoro substituted hydroxyapatite (FHAp) samples were prepared by a cyclic pH method. Both calcined and uncalcined samples were subjected to elemental analysis (F, Ca, P) and X-ray diffraction (XRD) analysis to verify composition and phase purity. Good correlation between a-axis parameters and fluoride ion content was found for calcined samples, however, for uncalcined samples the fluoride ion content was higher than estimated from the a-axis values. Fourier transform infra red (FT-IR) spectroscopy analysis of the calcined samples showed OH band shifts and splitting in accordance with F-HO interactions affecting the OH vibration. We conclude that the OH libration (620–780 cm-1 range) is more suited for estimation of fluoride ion content than the OH stretching. In contrast, uncalcined samples all displayed FT-IR spectra similar to that of hydroxyapatite (HAp) despite the presence of fluoride ions (18–73%). FT-IR emission spectroscopy was used to probe the changes occurring in the FT-IR spectra of HAp and FHAp samples upon heating. Interpretation of the spectral changes occurring during heating to 1,000 °C and subsequent cooling is given. Room temperature spectra of samples heated to various temperatures was used to determine the temperature necessary to produce FT-IR spectra displaying the expected OH bands. A model accounting for the combined observations is proposed.







Similar content being viewed by others
References
J. C. ELLIOTT “Structure and chemistry of the apatites and other calcium orthophosphates” in Studies in Inorganic Chemistry 18 (Elsevier, Amsterdam, 1994)
G. RIZZI, R. GROPPETTI, L. SALVARANI and A. A. SCRIVANI, Key Eng. Mater. 218–220 (2002) 93
M. WEI, J. H. EVANS and E. WENTRUP-BYRNE, J. Australasian Ceram. Soc. 36 (2000) 47
F. FREUND and R. M. KNOBEL, J. Chem. Soc. Dalton Trans. (1977) 1136
A. BAUMER, M. GANTEAUME and W. E. KLEE, Bull. Mineral. 108 (1985) 145
R. A. YOUNG, W. Van der LUGT and J. C. ELLIOTT, Nature, 223 (1969) 729
G .C. MAITI, Ind. J. Chem. 29A (1990) 402
G .C. MAITI, Ind. J. Chem. 31A (1992) 276
L. GINESTE, M. GINESTE, X. RANZ, A. ELLEFTERION, A. GUILHAM, N. ROUGUET and P. FRAYSSINET, J. Biomed. Mater. Res. (Appl. Biomater.) 48 (1999) 224
A. BIGI, E. FORESTI, A. RIPAMONTI and N. ROVERI, J. Inorg. Biochem. 27 (1986) 31
A. KRAJEWSKI, A. RAVAGLIOLI, N. ROVERI, A. BIGI and E. FORESTI, J. Mater. Sci. 25 (1990) 3203
L .J. JHA, S. M. BEST, J. C. KNOWLES, I. REHMAN, J. D. SANTOS and W. BONFIELD, J. Mater. Sci. Mater. Med. 8 (1997) 185
I. MANJUBALA, M. SIBAKUMAR and S. NAJMA NIKKATH, J. Mater. Sci. 36 (2001) 5481
H. QU and M. WEI, J. Mater. Sci. Mater. Med. 16 (2005) 129
M. WEI, J. H. EVANS, T. BOSTROM and L. GRØNDAHL, J. Mater. Sci. Mater. Med. 14 (2003) 311
E. J. DUFF, Chem. Ind. 8 (1974) 349
E. J. DUFF and J. L. STUART, Anal. Chim. Acta 52 (1970) 155
A. M. VASSALLO, P. A. COLE-CLARKE, L. S. K. PANG and A. J. PALMISANO, Appl. Spectrosc. 46 (1992) 73
L. RINTOUL, H. PANAYIOTOU, S. KOKOT, G. GEORGE, G. CASH, R. FROST, T. BUI and P. FREDERICKS, Analyst, 123 (1998) 571
E. J. DUFF, Archs. Oral Biol. 20 (1975) 763
B .O. FOWLER, Inorg. Chem. 13 (1974) 207
R.A. YOUNG and D. W. HOLCOMB, Calcif. Tissue Int. 34 (1982) S17
D .G. A. NELSON and B. E. WILLIAMSON, Aust. J. Chem. 35 (1982) 715
F. FREUND, Inorg. Nucl. Chem. Lett. 13 (1977) 57
F. FREUND, “Ceramics and thermal transformations of minerals”, in “The Infrared Spectra of Minerals” edited by V. V. Farmer, Mineralogical Society, London (1974) pp. 465–482
H. NISHIKAWA, Mater. Lett. 50 (2001) 354
K. TÕNSUAADU, M. PELD and V. BEDER, J. Therm. Anal. Cal. 72 (2003) 363
N.W. CANT, J. A. S. BETT, R. W. GEOFFREY and W. K. HALL, Spectrochim. Acta 27A (1971) 425
I. REISNER and W. E. KLEE, Spectrochim. Acta 38A (1982) 899
J. SHI, A. KLOCKE, M. ZHANG and U. BISMAYER, Am. Mineral. 88 (2003) 1866
Y. PAN, Phosphorous, Sulfur and Silicon 144–146 (1999) 413
Acknowledgements
Mr. T. Raftery, X-ray analysis facility QUT, is greatly acknowledged for XRD measurements and expert assistance in XRD analysis. Dr Wayde Martens is thanked for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rintoul, L., Wentrup-Byrne, E., Suzuki, S. et al. FT-IR spectroscopy of fluoro-substituted hydroxyapatite: strengths and limitations. J Mater Sci: Mater Med 18, 1701–1709 (2007). https://doi.org/10.1007/s10856-007-3052-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-007-3052-3