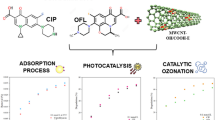
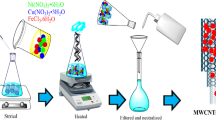
Abstract
Cold plasma-driven (multi-walled carbon nanotubes) MWCNTs as a hybrid process were operated to eliminate antibiotics from contaminated aqueous solutions. XRD, BET-BJH, FTIR, Raman, FESEM, and TEM analyses were performed to determine the catalyst characteristics. The results demonstrated that using the MWCNT as a catalyst enhanced the formation of hydroxyl radicals, ozone molecules, and active oxygen groups on the surface. Also, by applying plasma, the dispersion of catalyst components in the solution medium increases; the oxidation process in the solution occurs more actively and it leads to further decomposition of ozone molecules, hydroxide, and peroxide radicals; the specific surface area increases; and so, the adsorption of pollutant molecules increases, MWCNT provides a suitable position to create more micro-discharges, and as a result, the number of active species increases, and MWCNT increases the lifetime of reactive species. So, the excellent performance of the hybrid process obtained for the degradation of aqua solutions of ciprofloxacin (CIP) (90.6%), ofloxacin (OFL) (83.0%), and levofloxacin (LVO) (72.4%) in concentration of 80 mg/L for 60 min. However, the percentage removal rate decreased with increase in initial antibiotic concentration. In the ciprofloxacin concentrations of 80, 110 and 170 mg/L, the efficiency degradations were 90.6, 65.7, and 54.0%, respectively. By increasing the amount of MWCNT catalyst, the removal performance improves. The percentage removal in the presence of MWCNT at the levels of 0.25, 0.5 and 2.5 g/L was equal to 85.0, 90.6, and 98.7%, respectively. The use of MWCNT activates the oxidation process and increases the specific surface area. As the amount of catalyst increases, more substrate is provided for the generation of reactive oxygen species to stimulate the contaminant removal. Examination of different pHs showed that the effect of this parameter depends on the molecular structure between the particles. The results show that the removal percentage of CIP (ciprofloxacin) is 55.6, 90.6 and 86.5% at pH 2, 6 and 11, respectively. And, the study of gas type in contact with the aqueous shows plasma in percent oxygen has high efficiency (90.6%) rather than air (40.7%) after 60 min. Also, for reusability, the used catalyst (MWCNT) was investigated in four cycles, and finally, the mechanism of reactions that occurred in the solution was proposed.


















Similar content being viewed by others
Data availability
The authors don't have permission to share data.
References
A.B. Sar, E.G. Shabani, M. Haghighi, M. Shabani, J. Taiwan Inst. Chem. Eng. 132, 104131 (2022)
N. Mikaeeli, M. Haghighi, E. Fatehifar, M. Shabani, Appl. Surf. Sci. 572, 151433 (2022)
M. Jodeyri, M. Haghighi, M. Shabani, J. Mater. Sci. Mater. Electron. 30, 13877–13894 (2019)
Z. Zhong, W. Chen, X. Chen, J. Li, H. Yang, L. Zhang, P. Yang, J. Mater. Sci. Mater. Electron. 35, 244 (2024)
L.S. Alqarni, M.D. Alghamdi, H. Alhussain, N.Y. Elamin, K.K. Taha, A. Modwi, J. Mater. Sci. Mater. Electron. 35, 239 (2024)
Y. Li, L. Li, R. Zhang, Z. Ying, Y. Zhou, W. Wu, G. Wang, J. Mater. Sci. Mater. Electron. 35, 236 (2024)
Z. Abdollahizadeh, M. Haghighi, M. Shabani, Sep. Purif. Technol. 278, 119574 (2021)
M. Shabani, M. Haghighi, D. Kahforoushan, S. Heidari, J. Taiwan Inst. Chem. Eng. 96, 243–255 (2019)
M. Jodeyri, M. Haghighi, M. Shabani, Ultrason. Sonochem. 54, 220–232 (2019)
M. Dang, Y. Guo, Y. Tian, J. Mater. Sci. Mater. Electron. 35, 221 (2024)
L. Yaqi, C. Ling, D. Yimin, L. Qi, F. Chengqian, W. Zhiheng, C. Ling, L. Bo, Z. Yue-Fei, L. Yan, W. Li, J. Mater. Sci. Mater. Electron. 35, 215 (2024)
H. Kumari, Sonia, S. Chahal, Suman, P. Kumar, A. Kumar, R. Parmar, J. Mater. Sci. Mater. Electron. 35, 212 (2024)
A. Alam, W.U. Rahman, Z.U. Rahman, S.A. Khan, Z. Shah, K. Shaheen, H. Suo, M.N. Qureshi, S.B. Khan, E.M. Bakhsh, K. Akhtar, J. Mater. Sci. Mater. Electron. 33, 4255–4267 (2022)
Q. Wei, W. Li, C. Jin, Y. Chen, L. Hou, Z. Wu, Z. Pan, Q. He, Y. Wang, D. Tang, J. Rare Earths 40, 595–604 (2022)
S. Singh, P. Kaur, V. Kumar, K.B. Tikoo, S. Singhal, J. Rare Earths 39, 781–789 (2021)
A. James, J.D. Rodney, A. Manojbabu, S. Joshi, L. Rao, B.R. Bhat, N.K. Udayashankar, J. Mater. Sci. Mater. Electron. 35, 190 (2024)
N. Mohseni, M. Haghighi, M. Shabani, Process. Saf. Environ. Prot. 168, 668–688 (2022)
A. Sokhansanj, M. Haghighi, M. Shabani, J. Mol. Liq. 371, 121024 (2023)
S.R. Bavaji, A.J. Ahamed, J. Mater. Sci. Mater. Electron. 35, 147 (2024)
A. Najafidoust, M. Haghighi, E. Abbasi Asl, H. Bananifard, Sep. Purif. Technol. 221, 101–113 (2019)
M. Shabani, M. Haghighi, D. Kahforoushan, A. Haghighi, Sol. Energy Mater. Sol. Cells 193, 335–350 (2019)
H. Tong, T. He, R. He, G. Chen, D. Qian, D. Wu, J. Mater. Sci. Mater. Electron. 35, 144 (2024)
M. Gavahian, N. Pallares, F. AlKhawli, E. Ferrer, F.J. Barba, Trends Food Sci. Technol. 106, 209–218 (2020)
H.A. Raza, M. Shafiq, M. Naeem, M.Y. Naz, ChemistrySelect 4, 5348–5354 (2019)
Y. Gazal, C. Dublanche-Tixier, C. Chazelas, M. Colas, P. Carles, P. Tristant, Thin Solid Films 600, 43–52 (2016)
S. Samal, J. Clean. Prod. 142, 3131–3150 (2017)
H. Sobral, R. Sanginés, Spectrochim. Acta Part B 94–95, 1–6 (2014)
J.W. Borchert, U. Zschieschang, F. Letzkus, M. Giorgio, R.T. Weitz, M. Caironi, J.N. Burghartz, S. Ludwigs, H. Klauk, Sci. Adv. 6, eaaz5156 (2020)
T. Shimizu, Y. Ikehara, J. Phys. D Appl. Phys. 50, 503001 (2017)
P. Ranieri, N. Sponsel, J. Kizer, M. Rojas-Pierce, R. Hernández, L. Gatiboni, A. Grunden, K. Stapelmann, Plasma Processes Polym. 18, 2000162 (2021)
Y. Zhang, Z. Wei, Y. Zhu, S. Tao, M. Chen, Z. Zhang, Z. Jiang, W. Shangguan, J. Rare Earths 41, 789–800 (2023)
J.O. Tijani, K.O. Badmus, O. Pereao, O. Babajide, C. Zhang, T. Shao, E. Sosnin, V. Tarasenko, O.O. Fatoba, Int. J. Environ. Res. Public Health 18, 1683 (2021)
M.Y. Naz, S. Shukrullah, A. Ghaffar, N.U. Rehman, M. Sagir, Synthesis and reactivity in inorganic, metal-organic, and nano-metal. Chemistry 46, 104–109 (2016)
E.S. Massima Mouele, J.O. Tijani, K.O. Badmus, O. Pereao, O. Babajide, C. Zhang, T. Shao, E. Sosnin, V. Tarasenko, O.O. Fatoba, K. Laatikainen, L.F. Petrik, J. Hazard. Mater. 417, 125481 (2021)
J. Zeng, B. Yang, X. Wang, Z. Li, X. Zhang, L. Lei, Chem. Eng. J. 267, 282–288 (2015)
M. Rashid, M. Chowdhury, M. Talukder, J. Environ. Chem. Eng. 8, 104504 (2020)
S. Suarez, E. Ramos-Moore, F. Mücklich, Carbon 51, 404–409 (2013)
N. Garmendia, A. Arteche, A. García, I. Bustero, I. Obieta, J. Compos. Mater. 43, 247–256 (2009)
F. Pourfayaz, Y. Mortazavi, A.-A. Khodadadi, S.H. Jafari, S. Boroun, M.V. Naseh, Appl. Surf. Sci. 295, 66–70 (2014)
Y.G. Denisenko, M.S. Molokeev, A.S. Oreshonkov, A.S. Krylov, A.S. Aleksandrovsky, N.O. Azarapin, O.V. Andreev, I.A. Razumkova, V.V. Atuchin, Crystals 11, 1027 (2021)
N. Golovnev, M. Molokeev, S. Vereshchagin, V. Atuchin, J. Coord. Chem. 66, 4119–4130 (2013)
N.N. Golovnev, M.S. Molokeev, S.N. Vereshchagin, V.V. Atuchin, M.Y. Sidorenko, M.S. Dmitrushkov, Polyhedron 70, 71–76 (2014)
N. Sakakibara, K. Inoue, S. Takahashi, T. Goto, T. Ito, K. Akada, J. Miyawaki, Y. Hakuta, K. Terashima, Y. Harada, Phys. Chem. Chem. Phys. 23, 10468–10474 (2021)
S. Chen, H. Wang, M. Shi, H. Ye, Z. Wu, Environ. Sci. Technol. 52, 8568–8577 (2018)
Z. Jia, M.B. Amar, D. Yang, O. Brinza, A. Kanaev, X. Duten, A. Vega-González, Chem. Eng. J. 347, 913–922 (2018)
M. Zhu, S. Hu, F. Wu, H. Ma, S. Xie, C. Zhang, J. Phys. D Appl. Phys. 55, 225207 (2022)
A.A. Aryee, E. Dovi, X. Shi, R. Han, Z. Li, L. Qu, Colloids Surf. A 615, 126260 (2021)
G. Crini, E. Lichtfouse, Environ. Chem. Lett. 17, 145–155 (2019)
J. Chakraborty, I. Nath, C. Jabbour, N. Aljammal, S. Song, C.-M. Kao, P.M. Heynderickx, F. Verpoort, J. Hazard. Mater. 398, 122928 (2020)
M.J. Lima, C.G. Silva, A.M. Silva, J.C. Lopes, M.M. Dias, J.L. Faria, Chem. Eng. J. 310, 342–351 (2017)
A. Huang, D. Zhi, H. Tang, L. Jiang, S. Luo, Y. Zhou, Sci. Total. Environ. 720, 137560 (2020)
E.A. Serna-Galvis, A.M. Botero-Coy, D. Martínez-Pachón, A. Moncayo-Lasso, M. Ibáñez, F. Hernández, R.A. Torres-Palma, Water Res. 154, 349–360 (2019)
A. Ashiq, M. Vithanage, B. Sarkar, M. Kumar, A. Bhatnagar, E. Khan, Y. Xi, Y.S. Ok, Environ. Res. 197, 111091 (2021)
X. Chen, J. Wang, Chem. Eng. J. 395, 125095 (2020)
J.J.S. Alonso, N. El Kori, N. Melián-Martel, B. Del Río-Gamero, J. Environ. Manag. 217, 337–345 (2018)
D.A. Palacio, B.F. Urbano, B.L. Rivas, J. Water Process Eng. 46, 102582 (2022)
C. Fang, Q. Huang, Plasma Med. 8, 321–333 (2018)
C.A. Aggelopoulos, Chem. Eng. J. 428, 131657 (2022)
C. Aggelopoulos, S. Meropoulis, M. Hatzisymeon, Z. Lada, G. Rassias, Chem. Eng. J. 398, 125622 (2020)
P. Guerra, M. Kim, A. Shah, M. Alaee, S. Smyth, Sci. Total. Environ. 473, 235–243 (2014)
Acknowledgements
Financial supports for this study were provided by the Sahand University of Technology and the Iran National Science Foundation.
Funding
Funding was provided by Sahand University of Technology and Iran National Science Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This research is in compliance with ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Badi Sar, A., Haghighi, M., Ghareh Shabani, E. et al. Removal evolution of ciprofloxacin, ofloxacin, and levofloxacin contaminants via synergic influence of cold plasma-driven MWCNTs hybrid process: evaluation of operational parameters. J Mater Sci: Mater Electron 35, 959 (2024). https://doi.org/10.1007/s10854-024-12566-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12566-9