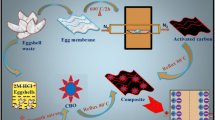
Abstract
The chicken eggshell has pores which deliver air to yolk and they are made from calcite crystals. To make this shell more porous by treating it with various solvents and carbonizing at different temperatures to use them as electrode material for energy storage devices. The eggshell waste-03 sample stands out among the other two in terms of crystallinity, crystallite size, and porosity according to X-ray diffraction (XRD), Brunauer–Emmett–Teller (BET) Surface area analyser, and Field Emission Scanning Electron Microscopout among the other two in terms of crystallinity, crystallite size, and porosity according to X-ray diffraction (XRD), Brunauer–Emmett–Teller (BET) Surface area analyser, and Field Emission Scanning Electron Microscopy (FESEM) results. The presence of elemental composition of calcium, carbon, and oxygen is revealed by the Energy Dispersive X-Ray (EDX) analysis and the intermolecular chemical interactions C=O, C–O and Ca–O are validated by Fourier Transform Infra-Red Spectroscopy (FTIR) analysis. In terms of practical utilization, the electrochemical performance studies reveal graphene oxide incorporated with eggshell waste-based supercapacitor electrode is 64.52% capacitance retention over 2500 GCD cycles with columbic efficiency of 96.02%. It is observed maximum specific capacitance value of 453 F/g at current density of 2 A/g from GCD and CV curve exhibits 397 F/g at scan rate of 10 mV/s, respectively. Hence, Eggshell waste with enhanced porous nature for carbonized materials is naturally occurring, affordable, and promising material for energy storage applications.









Similar content being viewed by others
Data availability
The research work carried out within the limitations of research policy and all data presented in the article do not have any problem.
References
H. Salvarli, M.S. Salvarli, in Renewable Energy - Resources, Challenges and Applications, ed. by M.A. Qubeissi, A. El-Kharouf, H.S. Soyhan (IntechOpen, 2020)
L. Zhang, H.B. Saydaliev, X. Ma, Renew. Energy 193, 991–1000 (2022)
Z.A. Arfeen, M.P. Abdullah, R. Hassan, B.M. Othman, A. Siddique, A.U. Rehman, U.U. Sheikh, Energy storage usages: engineering reactions, economic- technological values for electric vehicles-a technological outlook. Int. Trans. Electr. Energy Syst. 30(9), e12422 (2020)
G. Li, L. Sheng, T. Li, J. Hu, P. Li, K. Wang, Sol. Energy (2019). https://doi.org/10.1016/j.solener.2018.11.017
J.O.G. Posada, A.J. Rennie, S.P. Villar, V.L. Martins, J. Marinaccio, A. Barnes, J.P. Hall, Aqueous batteries as grid scale energy storage solutions. Renew. Sustain. Energy Rev. 68, 1174–1182 (2012)
M.A. Hannan, S.B. Wali, P.J. Ker, M.S.A. Rahman, M. Mansor, Ramachandara- murthy VK, Dong, Y Z, Battery energy-storage system: a review of technologies, optimization objectives, constraints, approaches, and out- standing issues. J. Energy Storage 42, 103023–103023 (2021)
A.A. Kebede, T. Kalogiannis, J.V. Mierlo, M. Berecibar, A comprehensive review of stationary energy storage devices for large scale renewable energy sources grid integration. Renew. Sustain. Energy Rev. 159, 112213 (2022)
M. Leng, C. Xia, Z. Zhou, X. Shen, J. Bi, C. Huang, A distinctive strategy of Sb doped quaternary oxide cathodes materials toward energy storage of electric equipment for sodium-ion batteries. Electrochim. Acta 441, 141867 (2023)
M. Leng, J. Bi, Z. Xing, W. Wang, X. Gao, J. Wang, Z. Qian, A new perspective on the composition-structure-property relationships on Nb/Mo/Cr-doped O3-type layered oxide as cathode materials for sodium-ion batteries. Chem. Eng. J. 413, 127824 (2021)
A.G. Olabi, M.A. Abdelkareem, T. Wilberforce, E.T. Sayed, Application of graphene in energy storage device-a review. Renew. Sustain. Energy Rev. 135, 110026 (2021)
S. Fang, D. Bresser, S. Passerini, Transit. Metal Oxid. Electrochem. Energy Storage (2022). https://doi.org/10.1002/9783527817252.ch4
Y. Cheng, J. Chen, Y. Chen, X. Ke, J. Li, Y. Yang, Z. Shi, Lithium host: advanced architecture components for lithium metal anode. Energy Storage Mater. 38, 276–298 (2021)
I. Hussain, S. Iqbal, C. Lamiel, A. Alfantazi, K. Zhang, Recent advances in oriented metal-organic frameworks for supercapacitive energy storage. J. Mater. Chem. A 10(9), 4475–4488 (2022)
I. Hadjipaschalis, A. Poullikkas, V. Efthimiou, Energy Eng. 120, 147–161 (2009)
J.C. Selvakumari, S.T. Nishanthi, J. Dhanalakshmi, M. Ahila, D.P. Padiyan, Bio-active synthesis of tin oxide nanoparticles using eggshell membrane for energy storage application. Appl. Surf. Sci. 441, 530–537 (2018)
L. Huang, J. Li, Z. Wang, Y. Li, X. He, Y. Yuan, Microwave absorption enhancement of porous C@ CoFe2O4 nanocomposites derived from eggshell membrane. Carbon 143, 507–516 (2019)
R.J.D. Silva, R.M. Lima, M.C.A.D. Oliveira, J.J. Alcaraz-Espinoza, C.P.D. Melo, H.P. Oliveira, Supercapacitors based on (carbon nanostructure)/PEDOT/ (eggshell membrane) electrodes. J. Electroanal. Chem. 856, 113658 (2020)
S.H. Kim, G. Kumar, W.H. Chen, S.K. Khanal, Renewable hydrogen pro- duction from biomass and wastes (ReBioH2–2020). Bioresour. Technol. 331, 125024 (2021)
L. Ma, R. Chen, Y. Hu, W. Zhang, G. Zhu, P. Zhao, Z. Jin, Nanoporous and lyophilic battery separator from regenerated eggshell membrane with effective suppression of dendritic lithium growth. Energy Storage Mater. 14, 258–266 (2018)
M. Minakshi, S. Higley, C. Baur, D.R. Mitchell, R.T. Jones, M. Fichtner, Cal- cined chicken eggshell electrode for battery and supercapacitor applications. RSC Adv. 9, 26981–26995 (2019)
N. Therdthai, A. Soontrunnarudrungsri, W. Khotchai, Modified eggshell powder using thermal treatment and its application in Ca-fortified dog biscuits. Heliyon 9(2), e13093 (2023)
A.K. Thakur, R. Sathyamurthy, Improving the potable water generation through tubular solar still using eggshell powder (bio-based energy source) as a natural energy storage material-an experimental approach. Environ. Sci. Pollut. Res. 29(27), 40903–40920 (2022)
L. Scala, N. Boleli, I.C. Ribeiro, L.T. Freitas, D. Macari, M, Pore size dis- tribution in chicken eggs as determined by mercury porosimetry. Braz. J. Poul. Sci. 2, 177–181 (2000)
J. Yang, Y. Jia, D. Hou, P. Wang, Z. Jin, H. Shang, T. Zhao, Na and Cl immobilization by size controlled calcium silicate hydrate nanometer pores. Constr. Build. Mater. 202, 622–635 (2019)
N.I. Zaaba, K.L. Foo, U. Hashim, S.J. Tan, W.W. Liu, C.H. Voon, Proc. Eng. (2017). https://doi.org/10.1016/j.proeng.2017.04.118
D.S.R. Krishna, A. Siddharthan, S.K. Seshadri, T.S. Kumar, A novel route for synthesis of nanocrystalline hydroxyapatite from eggshell waste. J. Mater. Sci. - Mater. Med. 18, 1735–1743 (2007)
D. Dollimore, P. Spooner, A.J.S.T. Turner, The BET method of analysis of gas adsorption data and its relevance to the calculation of surface areas. Surface Technol. 4(2), 121–160 (1976)
A. Jiménez, V. Rives, M.A. Vicente, Thermal study of the hydrocalumite- katoite-calcite system. Thermochim. Acta 713, 179242–179242 (2022)
A. Jiménez, R. Trujillano, V. Rives, M.A. Soria, L.M. Madeira, M.A. Vicente, CaAlFe-mixed metal oxides prepared from an aluminum salt-cake and their evaluation as CO2 sorbents at moderate temperature. Chem. Eng. J. 473, 145165 (2023)
F.C. Donnelly, F. Purcell-Milton, V. Framont, O. Cleary, P.W. Dunn, Y.K. Gun’ko, Synthesis of CaCO 3 nano-and micro-particles by dry ice carbonation. Chem. Commun. 53(49), 6657–6660 (2017)
C. Li, S. Zhao, X. Yao, L. He, S. Xu, X. Shen, Z. Yao, The catalytic mechanism of intercalated chlorine anions as active basic sites in MgAl- layered double hydroxide for carbonyl sulfide hydrolysis. Environ. Sci. Pollut. Res. 29, 10605–10616 (2022)
T. Schädle, B. Pejcic, B. Mizaikoff, Monitoring dissolved carbon dioxide and methane in brine environments at high pressure using IR-ATR spec- troscopy. Anal. Methods 8(4), 756–762 (2016)
Cahya M, Marfuah N (2014) Identification of calcium carbonate (CaCO3) characteristics from different kinds of poultry eggshells using x-ray diffraction (XRD) and fourier transformation infra-red (FTIR). 2014 international conference on physics and its applications (ICOPIA-14) pp 138–142
A.H. Shah, Y. Zhang, X. Xu, A.Q. Dayo, X. Li, S. Wang, W. Liu, Reinforcement of stearic acid treated egg shell particles in epoxy thermosets:structural, thermal and mechanical characterization. Materials 11(10), 1872–1872 (2018)
W. Ahmad, S. Sethupathi, Y. Munusamy, R. Kanthasamy, Catalyst 11, 295 (2021)
F.S. Murakami, P.O. Rodrigues, C.M.T.D. Campos, M.A.S. Silva, Physico- chemical study of CaCO3 from egg shells. Food Sci. Technol. 27, 658–662 (2007)
H. Wu, H. Wei, X. Yang, C. Jin, W. Sun, K. Deng, C. Sun, Spherical activated carbons derived from resin-microspheres for the adsorption of acetic acid. J. Environ. Chem. Eng. 11(2), 109394 (2023)
T.F. Tadros, J. Lyklema, Adsorption of potential-determining ions at the silica-aqueous electrolyte interface and the role of some cations. J. Electroanal. Chem. Interfac. Electrochem. 17(3–4), 80206–80208 (1968)
S.M. Naga, H.F. El-Maghraby, M. Sayed, E.A. Saad, Highly porous scaf- folds made of nanosized hydroxyapatite powder synthesized from eggshells. J. Ceram. Sci. Technol. 6(3), 237–244 (2015)
I.M. Rashid, M.A. Atiya, B.H. Hameed, Production of biodiesel from waste cooking oil using CaO-egg shell waste derived heterogeneous catalyst. Int. J. Sci. Res. 6(11), 94–103 (2015)
D. Karuppiah, R. Palanisamy, A. Ponnaiah, W.R. Liu, C.H. Huang, S. Rengapillai, S. Marimuthu, Eggshell-membrane-derived carbon coated on Li2FeSiO4 cathode material for Li-ion batteries. Energies 13(4), 786 (2020)
X. Meng, D. Deng, Trash to treasure: waste eggshells as chemical reactors for the synthesis of amorphous Co (OH) 2 nanorod arrays on various substrates for applications in rechargeable alkaline batteries and electrocatalysis. ACS Appl. Mater. Interfaces 9(6), 5244–5253 (2017)
D. Muthu, G.S. Kumar, M. Gowri, M. Prasath, V. Viswabaskaran, V.S. Kattimani, E.K. Girija, Rapid synthesis of eggshell derived hydroxyapatite with nanoscale characteristics for biomedical applications. Ceram. Int. 48(1), 1326–1339 (2022)
M. Pathak, S.R. Polaki, C.S. Rout, High performance asymmetric super- capacitors based on Ti 3 C 2 T x MXene and electrodeposited spinel NiCo2S4 nanostructures. RSC Adv. 12(17), 10788–10799 (2022)
R.M. Obodo, N.M. Shinde, U.K. Chime, S. Ezugwu, A.C. Nwanya, I. Ahmad, I.F. Ezema, Curr. Opin. Electrochem. (2020). https://doi.org/10.1016/j.coelec.2020.02.022
Y.F. Wang, S.J. Zou, W.P. Hu, F.F. Wu, J.X. Yang, Y.Y. Cen, J.K. Huang, Biomass-derived graphene-like carbon nanoflakes for advanced supercapac- itor and hydrogen evolution reaction. J. Alloy. Compd. 928, 167176–167176 (2022)
Y. Wang, Y. Song, Y. Xia, Electrochemical capacitors: mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 45(21), 5925–5950 (2016)
Y.N. Sudhakar, H. Hemant, S.S. Nitinkumar, P. Poornesh, M. Selvakumar, Green synthesis and electrochemical characterization of rGO-CuO nanocom- posites for supercapacitor applications. Ionics 23, 1267–1276 (2017)
X.F. Wang, Z. You, D.B. Ruan, A hybrid metal oxide supercapacitor in aqueous KOH electrolyte. Chin. J. Chem. 24(9), 1126–1132 (2006)
Y. Lin, H. Tian, J. Qian, M. Yu, T. Hu, U. Lassi, Z. Wu, Biocarbon-directed vertical δ-MnO2 nanoflakes for boosting lithium-ion diffusion kinetics. Mater. Today Chem. 26, 101023 (2022)
A. Ramadoss, G.S. Kim, S.J. Kim, Fabrication of reduced graphene oxide/TiO2 nanorod/reduced graphene oxide hybrid nanostructures as electrode materials for supercapacitor applications. CrystEngComm 15(47), 10222–10229 (2013)
W.T. Tsai, J.M. Yang, C.W. Lai, Y.H. Cheng, C.C. Lin, C.W. Yeh, Charac- terization and adsorption properties of eggshells and eggshell membrane. Biores. Technol. 97(3), 488–493 (2006)
Funding
We would like to thank DST-PURSE laboratory, Mangalore University for their FESEM facility and SC/ST cell, Mangalore University for providing fellowship to pursue research work.
Author information
Authors and Affiliations
Contributions
All authors are made contribution in their own to this article as follows; SDS: He has conducted all experimental measurements and data analysis. ABS: He has involved in the synthesis of material. SS: She has contributed to conduct characterization of material. VSP: He has involved to fabricate the supercapacitor electrode. GH: He has contributed to write a manuscript. VS: He has reviewed the manuscript. NYS: He has helped to grammar check of the manuscript. DH: Corresponding author contributed as a mentor to this work.
Corresponding author
Ethics declarations
Conflict of interest
Authors must disclose all relationships or interests that could have direct or potential influence or impart bias on the work. Although an author may not feel there is any conflict, disclosure of relationships and interests provides a more complete and transparent process, leading to an accurate and objective assessment of the work. Awareness of a real or perceived conflict of interest is a perspective to which the readers are entitled. This is not meant to imply that a financial relationship with an organization that sponsored the research or compensation received for consultancy work is inappropriate.
Ethical approval
To ensure objectivity and transparency in research and to ensure that accepted principles of ethical and professional conduct have been followed, authors should include information regarding sources of funding, potential conflicts of interest (financial or non-financial), informed consent if the research involved human participants, and a statement on welfare of animals if the research involved animals. The corresponding author should be prepared to collect documentation of compliance with ethical standards. The author will be held responsible for false statements or failure to fulfill the above-mentioned guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Suresh, D.S., Ba Shbil, A., Sharanappa, S. et al. Enhancement of crystallinity with porosity material through solvent and thermal treated eggshell waste for high-performance supercapacitor applications. J Mater Sci: Mater Electron 35, 330 (2024). https://doi.org/10.1007/s10854-024-12021-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-024-12021-9