Abstract
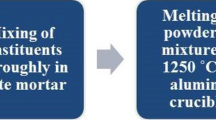
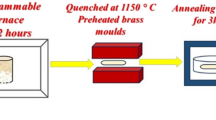
The quenching melt process was used to create transparent glass samples of the Fe and Eu-co-doped lithium borosilicate glasses. The structure of the glass sample affects the molar volume, optical band gap, and thermal characteristics (glass transitions, softening, and melting temperatures). According to Mössbauer spectroscopy, the Fe2+/(Fe2+ + Fe3+) ratio remains between 0.1 and 0.13, and the valence state of iron ions is not significantly altered with Eu2O3 presence. With increasing Eu2O3 content, magnetic susceptibility was shown to decrease. Energy levels determined by UV/Vis and NIR spectroscopy, located at 362, 380, 395, 414, 465, 533, 583, 590, 2092, and 2202 nm were assigned to 7F0 → 5D4, 7F0 → 5G2, 7F0 → 5L6, 7F0 → 5D3, 7F0 → 5D2, 7F0 → 5D1, and 7F0 → 5D0 electronic transitions, respectively. Five transition band emission spectra were identified using an excitation wavelength of 395 nm. It was observed that by increasing Fe3+, the intensity of the emission peak decreases.



















Similar content being viewed by others
Data availability
No data were used for the research described in the article.
References
H. Wen, P.A. Tanner, Optical properties of 3d transition metal ion-doped sodium borosilicate glass. J. Alloys Compd. 625, 328–335 (2015). https://doi.org/10.1016/j.jallcom.2014.11.094
A.A. Ali, H.M. Shaaban, A. Abdallah, Spectroscopic studies of ZnO borate-tellurite glass doped with Eu2O3. J. Mater. Res. Technol. 7(3), 240–247 (2018). https://doi.org/10.1016/j.jmrt.2017.06.008
P.S. Wong, M.H. Wan, R. Hussin, H.O. Lintang, S. Endud, Structural and luminescence studies of europium ions in lithium aluminium borophosphate glasses. J. Rare Earths 32(7), 585–592 (2014). https://doi.org/10.1016/S1002-0721(14)60112-5
C.R. Kesavulu et al., Optical spectroscopy and emission properties of Ho3+-doped gadolinium calcium silicoborate glasses for visible luminescent device applications. J. Non-Cryst. Solids 474, 50–57 (2017)
B.N.K. Reddy, B.D. Raju, K. Thyagarajan, R. Ramanaiah, Y.-D. Jho, B.S. Reddy, Optical characterization of Eu3+ ion doped alkali oxide modified borosilicate glasses for red laser and display device applications. Ceram. Int. 43(12), 8886–8892 (2017)
C.R. Kesavulu, H.J. Kim, S.W. Lee, J. Kaewkhao, E. Kaewnuam, N. Wantana, Luminescence properties and energy transfer from Gd3+ to Tb3+ ions in gadolinium calcium silicoborate glasses for green laser application. J. Alloys Compd. 704, 557–564 (2017)
D.D. Ramteke, H.C. Swart, R.S. Gedam, Electrochemical response of Nd3+ ions containing lithium borate glasses. J. Rare Earths 35(5), 480–484 (2017)
C.R. Kesavulu, H.J. Kim, S.W. Lee, J. Kaewkhao, N. Chanthima, Y. Tariwong, Physical, vibrational, optical and luminescence investigations of Dy3+-doped yttrium calcium silicoborate glasses for cool white LED applications. J. Alloys Compd. 726, 1062–1071 (2017)
P. Aryal et al., Optical and luminescence characteristics of Eu3+-doped B2O3:SiO2:Y2O3:CaO glasses for visible red laser and scintillation material applications. J. Rare Earths 36(5), 482–491 (2018). https://doi.org/10.1016/j.jre.2017.09.017
J. Liu et al., Non-alkali glass substrate with improved mechanical properties for display devices. J. Non-Cryst. Solids 524, 119610 (2019)
Y. Du, J. Yuan, S. Han, S. Yan, Y. Wang, D. Chen, Luminescence and scintillation properties of CuO-doped SiO2–B2O3–La2O3 glass. Opt. Mater. (Amst.) 96, 109363 (2019)
Z. Wang, W. Ni, Y. Jia, L. Zhu, X. Huang, Crystallization behavior of glass ceramics prepared from the mixture of nickel slag, blast furnace slag and quartz sand. J. Non-Cryst. Solids 356(31–32), 1554–1558 (2010)
X. Ren, W. Zhang, Y. Zhang, P. Zhang, J. Liu, Effects of Fe2O3 content on microstructure and mechanical properties of CaO–Al2O3–SiO2 system. Trans. Nonferr. Met. Soc. China 25(1), 137–145 (2015)
B.O. Mysen, Redox equilibria and coordination of Fe2+ and Fe3+ in silicate glasses from 57Fe Mossbauer spectroscopy. J. Non-Cryst. Solids 95, 247–254 (1987)
Q. Yu, C. Yan, D. Yong, Y. Feng, D. Liu, Y. Bin, Effect of Fe2O3 on non-isothermal crystallization of CaO–MgO–Al2O3–SiO2 glass. Trans. Nonferr. Met. Soc. China 25(7), 2279–2284 (2015)
P.M.V. Teja, A.R. Babu, P.S. Rao, R. Vijay, D.K. Rao, Structural changes in the ZnF2–Bi2O3–GeO2 glass system doped with Fe2O3 by spectroscopic and dielectric investigations. J. Phys. Chem. Solids 74(7), 963–970 (2013)
P.R. Rao, L. Pavić, A. Moguš-Milanković, V.R. Kumar, I.V. Kityk, N. Veeraiah, Electrical and spectroscopic properties of Fe2O3 doped Na2SO4–BaO–P2O5 glass system. J. Non-Cryst. Solids 358(23), 3255–3267 (2012)
M.T. Nayak, J.A.E. Desa, P.D. Babu, Magnetic and spectroscopic studies of an iron lithium calcium silicate glass and ceramic. J. Non-Cryst. Solids 484(December 2017), 1–7 (2018). https://doi.org/10.1016/j.jnoncrysol.2017.12.050
A. Bhogi, P. Kistaiah, Spectroscopic properties of alkali alkaline earth borate glasses doped with Fe3+ ions. J. Aust. Ceram. Soc. 56(1), 127–138 (2020)
H.D. Shashikala, N.K. Udayashankar et al., Influence of Fe3+ ions on optical, structural, thermal and mechanical properties of Li2O–Na2O–K2O–ZnO–B2O3 based glass system. Ceram. Int. 46(4), 5213–5222 (2020)
X. He et al., Glass forming ability, structure and properties of Cr2O3–Fe2O3 co-doped MgO–Al2O3–SiO2–B2O3 glasses and glass-ceramics. J. Non-Cryst. Solids 529(September 2019), 2–9 (2020). https://doi.org/10.1016/j.jnoncrysol.2019.119779
S.P. Singh, R.P.S. Chakradhar, J.L. Rao, B. Karmakar, EPR, FTIR, optical absorption and photoluminescence studies of Fe2O3 and CeO2 doped ZnO–Bi2O3–B2O3 glasses. J. Alloys Compd. 493(1–2), 256–262 (2010)
I. Bulus, M. Isah, M.E. Garba, R. Hussin, S.A. Dalhatu, Influence of Eu3+ dopant on physical and optical properties of lithium-borosulfophosphate glasses. Niger. J. Technol. Dev. 15(4), 121 (2019). https://doi.org/10.4314/njtd.v15i4.3
S. Dalal et al., Effect of substituting iron on structural, thermal and dielectric properties of lithium borate glasses. Mater. Res. Bull. 70, 559–566 (2015)
R. Naik, S.C. Prashantha, H. Nagabhushana, K.M. Girish, Electrochemical, photoluminescence and EPR studies of Fe3+ doped nano Forsterite: effect of doping on tetra and octahedral sites. J. Lumin. 197(December 2017), 233–241 (2018). https://doi.org/10.1016/j.jlumin.2018.01.051
N. Wantana, E. Kaewnuam, N. Chanlek, H.J. Kim, J. Kaewkhao, Influence of Gd3+ on structural and luminescence properties of new Eu3+-doped borate glass for photonics material. Radiat. Phys. Chem. 207, 110812 (2023)
S. Kaewjaeng et al., Effect of Gd2O3 on the radiation shielding, physical, optical and luminescence behaviors of Gd2O3–La2O3–ZnO–B2O3–Dy2O3 glasses. Radiat. Phys. Chem. 185, 109500 (2021)
W. Rittisut et al., New developments in the Gd3+/Sm3+ ions doped lithium aluminum borate glasses of luminescent materials for lighting applications. Radiat. Phys. Chem. 201, 110443 (2022)
Y. Zhang et al., Tunable emission from green to red in the GdSr2AlO5:Tb3+, Eu3+ phosphor via efficient energy transfer. RSC Adv. 8(7), 3530–3535 (2018)
R. Vijayakumar, K. Maheshvaran, V. Sudarsan, K. Marimuthu, Concentration dependent luminescence studies on Eu3+ doped telluro fluoroborate glasses. J. Lumin. 154, 160–167 (2014). https://doi.org/10.1016/j.jlumin.2014.04.022
J. Rajagukguk, J. Kaewkhao, M. Djamal, R. Hidayat, Suprijadi, Y. Ruangtaweep, Structural and optical characteristics of Eu3+ ions in sodium–lead–zinc–lithium-borate glass system, J. Mol. Struct. 1121, 180–187 (2016). https://doi.org/10.1016/j.molstruc.2016.05.048
M.S. Gaafar, F.H. El-Batal, M. El-Gazery, S.A. Mansour, Effect of doping by different transition metals on the acoustical properties of alkali borate glasses. Acta Phys. Pol. A 115(3), 671–678 (2009). https://doi.org/10.12693/APhysPolA.115.671
T. Togashi, T. Honma, K. Shinozaki, T. Komatsu, Electrochemical performance as cathode of lithium iron silicate, borate and phosphate glasses with different Fe2+ fractions. J. Non-Cryst. Solids 436, 51–57 (2016). https://doi.org/10.1016/j.jnoncrysol.2016.02.001
E.A. Elkelany, M.A. Hassan, A. Samir, A.M. Abdel-Ghany, H.H. El-Bahnasawy, M. Farouk, Optical and Mössbauer spectroscopy of lithium tetraborate glass doped with iron oxide. Opt. Mater. (Amst.) (2021). https://doi.org/10.1016/j.optmat.2020.110744
Z.A. Alrowaili et al., The impact of Fe2O3 on the dispersion parameters and gamma/fast neutron shielding characteristics of lithium borosilicate glasses. Optik (Stuttg.) 249(September 2021), 168259 (2022). https://doi.org/10.1016/j.ijleo.2021.168259
M.K. Halimah et al., Optical properties of lithium borate glass (Li2O)x(B2O3)1–x (Sifat Optik Kaca Litium Borat (Li2O)x(B2O3)1–x). Sains Malay. 43(6), 899–902 (2014)
P.P. Pawar, S.R. Munishwar, R.S. Gedam, Eu2O3 doped bright orange–red luminescent lithium alumino-borate glasses for solid state lighting. J. Lumin. 200(October 2017), 216–224 (2018). https://doi.org/10.1016/j.jlumin.2018.04.026
T. Liu et al., Effect of Fe2O3 doping on structure, physical–mechanical properties and luminescence performance of magnesium–aluminum–silicon based glass-ceramics. Ceram. Int. 46(18), 28851–28859 (2020). https://doi.org/10.1016/j.ceramint.2020.08.051
S. Rakpanich, N. Wantana, Y. Ruangtaweep, J. Kaewkhao, Eu3+ doped borosilicate glass for solid-state luminescence material. J. Met. Mater. Miner. 27(1), 35–38 (2017). https://doi.org/10.14456/jmmm.2017.5
F. Gharghar, S. El-Jadal, The influence of iron oxide on the structural and optical properties of borosilicate. J. Appl. Sci. 9, 33–46 (2022)
Hirdesh, A. Khanna, Structural, thermal and photoluminescent properties of Eu2O3–Li2O–TeO2 glasses, J. Lumin. 204(August), 319–326 (2018). https://doi.org/10.1016/j.jlumin.2018.08.022
S. Singh, G. Kalia, K. Singh, Effect of intermediate oxide (Y2O3) on thermal, structural and optical properties of lithium borosilicate glasses. J. Mol. Struct. 1086, 239–245 (2015). https://doi.org/10.1016/j.molstruc.2015.01.031
Z. Luo, C. Qin, Y. Wu, W. Xu, S. Zhang, A. Lu, Structure and properties of Fe2O3-doped 50Li2O–10B2O3–40P2O5 glass and glass–ceramic electrolytes. Solid State Ion. 345(June 2019), 115177 (2020). https://doi.org/10.1016/j.ssi.2019.115177
P.P. Pawar, S.R. Munishwar, S. Gautam, R.S. Gedam, Physical, thermal, structural and optical properties of Dy3+ doped lithium alumino-borate glasses for bright W-LED. J. Lumin. 183, 79–88 (2017). https://doi.org/10.1016/j.jlumin.2016.11.027
C.L. Justino de Lima, F.A. Veer, H. Zhang, F. França de Mendonça Filho, O. Copuroglu, R. Nijsse, “Thermal, optical and mechanical properties of new glass compositions containing fly ash”, Glass Technol. Eur. J. Glass Sci. Technol. A 62(3), 96–108 (2021). https://doi.org/10.13036/17533546.62.3.007
E. Bykova et al., High-pressure behavior of Fe2O3. Acta Crystallogr. A 70(a1), C49 (2014). https://doi.org/10.1107/s2053273314099501
S.M. Salman, S.N. Salama, E.A. Mahdy, The crystallization process and chemical durability of glass–ceramics based on the Li2O–B2O3 (Fe2O3)–SiO2 (GeO2) system. Ceram. Int. 39(6), 7185–7192 (2013). https://doi.org/10.1016/j.ceramint.2013.02.063
Y. Xu et al., Impacts of substitution of Fe2O3 for SiO2 on structure and properties of borosilicate glasses containing MoO3. J. Non-Cryst. Solids 599(July 2022), 121982 (2023). https://doi.org/10.1016/j.jnoncrysol.2022.121982
M. Środa, S. Świontek, W. Gieszczyk, P. Bilski, The effect of lithium fluoride on the thermal stability and thermoluminescence properties of borosilicate glass and glass–ceramics. J. Eur. Ceram. Soc. 40(2), 472–479 (2020). https://doi.org/10.1016/j.jeurceramsoc.2019.09.037
M. Malik, S. Dagar, A. Hooda, A. Agarwal, S. Khasa, Effect of magnetic ion, Fe3+ on the structural and dielectric properties of oxychloro bismuth borate glasses. Solid State Sci. (2020). https://doi.org/10.1016/j.solidstatesciences.2020.106491
V.P. Manjari, C. Rama, C. Venkata, S. Muntaz, Y.P. Reddy, R.V.S.S.N. Ravikumar, Synthesis and characterization of undoped and Fe(III) ions doped NaCaAlPO4F3 phosphor. J. Lumin. 145, 324–329 (2014). https://doi.org/10.1016/j.jlumin.2013.07.052
M.M. Mohan, L.R. Moorthy, D. Ramachari, C.K. Jayasankar, Spectroscopic investigation and optical characterization of Eu3+ ions in K-Nb–Si glasses. Spectrochim. Acta A 118, 966–971 (2014). https://doi.org/10.1016/j.saa.2013.09.094
S.A. Loureno et al., Eu3+ photoluminescence enhancement due to thermal energy transfer in Eu2O3-doped SiO2B2O 3PbO2 glasses system. J. Lumin. 131(5), 850–855 (2011). https://doi.org/10.1016/j.jlumin.2010.11.028
M. Secu, C.E. Secu, Processing and optical properties of Eu-doped chloroborate glass–ceramic. Crystals 10(12), 1–12 (2020). https://doi.org/10.3390/cryst10121101
P. Ramesh et al., Compositional dependence of red photoluminescence of Eu3+ ions in lead and bismuth containing borate glasses. Solid State Sci. 107(July), 106360 (2020). https://doi.org/10.1016/j.solidstatesciences.2020.106360
N.A.S. Omar, Y.W. Fen, K.A. Matori, M.H.M. Zaid, N.F. Samsudin, Structural and optical properties of Eu3+ activated low cost zinc soda lime silica glasses. Results Phys. 6(September), 640–644 (2016). https://doi.org/10.1016/j.rinp.2016.09.007
Q. Huang, T. Liu, X. Shen, X. Li, A. Lu, Y. Gu, Characterization of Fe2O3 doping on structure, optical and luminescence properties of magnesium aluminosilicate-based glasses. J. Non-Cryst. Solids (2021). https://doi.org/10.1016/j.jnoncrysol.2021.120786
K.S. Shaaban, B.M. Alotaibi, N. Alharbiy, A.F.A. El-Rehim, Fabrication of lithium borosilicate glasses containing Fe2O3 and ZnO for FT-IR, UV–Vis–NIR, DTA, and highly efficient shield. Appl. Phys. A (2022). https://doi.org/10.1007/s00339-022-05474-4
M. Al Mahalawy, H. Farouk, A. Ratep, I. Kashif, Impact of bismuth concentration on the fluorescence properties of the bismuth borosilicate glasses. Opt. Quantum Electron. 53(7), 1–22 (2021). https://doi.org/10.1007/s11082-021-02966-0
M. Romero-Ferez, J.M. Rincón, C.J.R. González Oliver, C. D’Ovidio, D. Esparza, Magnetic properties of glasses with high iron oxide content. Mater. Res. Bull. 36(7–8), 1513–1520 (2001). https://doi.org/10.1016/S0025-5408(01)00630-4
M.D. Dyar, A review of Mössbauer data on inorganic glasses: the effects of composition on iron valency and coordination. Am. Mineral. 70(3–4), 304–316 (1985)
L. Skuja, Optically active oxygen-deficiency-related centers in amorphous silicon dioxide. J. Non-Cryst. Solids 239, 16–48 (1998). https://doi.org/10.1016/S0022-3093(98)00720-0
D. Ehrt, Photoluminescence in glass and glass ceramics photoluminescence in glasses and glass ceramics. IOP Conf. Ser. Mater. Sci. Eng. 2, 012001 (2009). https://doi.org/10.1088/1757-899X/2/1/012001
X. Guo, Q. Pan, X. Song, Q. Guo, S. Zhou, J. Qiu, G. Dong, Embedding carbon dots in Eu3+-doped metal–organic framework for label-free ratiometric fluorescence detection of Fe3+ ions. J. Am. Ceram. Soc. 104, 886–895 (2021). https://doi.org/10.1111/jace.17477
Y. Zheng, X. Wang, Z. Guan, J. Deng, X. Liu, H. Li, P. Zhao, Application of CD and Eu3+ dual emission MOF colorimetric fluorescent probe based on neural network in Fe3+ detection. Part. Part. Syst. Charact. (2022). https://doi.org/10.1002/ppsc.202200124
P. Li, X. Chen, C. Guo, H. Zou, Z. Chen, B. Liu, A logic fluorescent chemosensor based on Eu3+ functionalized Cd-MOFs for sensing Fe3+ and Cu2+ synchronously. Eur. J. Inorg. Chem. 26(3), e202200576 (2023). https://doi.org/10.1002/ejic.202200576
M. Murali Mohan, L. Rama Moorthy, D. Ramachari, C.K. Jayasankar, Spectroscopic investigation and optical characterization of Eu3+ ions in K-Nb–Si glasses. Spectrochim. Acta A 118, 966–971 (2014). https://doi.org/10.1016/j.saa.2013.09.094
N. Kiran, Eu3+ ion doped sodium–lead borophosphate glasses for red light emission. J. Mol. Struct. 1065, 93–98 (2014). https://doi.org/10.1016/j.molstruc.2014.02.047
E.M. Ahmed, E.A. Gaml, Effect of Pb/B ion replacement in B2O3·PbO·Fe2O3·Na2O glasses: optical, magnetic, and fluorescence properties. Opt. Mater. (Amst.) 116(April), 111075 (2021). https://doi.org/10.1016/j.optmat.2021.111075
Q. Cai, F. Zhou, N. Yang, H. Xu, S. Baccaro, A. Cemmi, M. Falconieri, G. Chen, Enhanced and shortened Mn2+ emissions by Cu+ co-doping in borosilicate glasses for W-LEDs. Opt. Mater. Express 5(1), 51 (2015). https://doi.org/10.1364/ome.5.000051
Acknowledgements
M.A.T. and M.L.M are staff members of CONICET (Argentina). Asmaa Ratep acknowledges TWAS Fellowship for partial financial support. And the authors thank Material Science and Glass Research Lab., Physics Department, Faculty of Science, AL-AZHAR University for providing the measurement facilities.
Funding
No funding for research.
Author information
Authors and Affiliations
Contributions
AR: Investigation, Writing—original draft, Methodology, Formal analysis. MLM: Investigation, Writing—original draft, Methodology, Formal analysis. MAT: Writing—review and editing, administration, Formal analysis, Investigation. IK: Writing—review and editing, Administration, Formal analysis, Investigation.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This paper meets the ethical standards of this journal.
Informed consent
All authors agree with the review of this paper in this journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kashif, I., Montes, M.L., Taylor, M.A. et al. Influence of Fe substitution on the Eu-doped lithium borosilicate glass system's physical, thermal, magnetic, and luminescent properties. J Mater Sci: Mater Electron 35, 273 (2024). https://doi.org/10.1007/s10854-023-11894-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-11894-6