Abstract
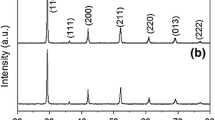
The development of SOFCs aims to reduce the operating temperature of the cells, maintaining the same level of efficiency with protonic conduction electrolytes, as they have low activation energy. In this context, comprehending the properties of these materials and devising methods that facilitate the production of dense ceramics are crucial aspects of this research. To address these issues, we added Fe2O3, Mn2O3 and ZnO as sintering aids to investigate their influence on BaCe0.2Zr0.7Y0.1O3-δ obtained by Pechini method. The sintering occurred via liquid phase sintering, resulting in densities higher than 96%. The barium carbonate reminiscent from calcination reacted with Fe and Mn arising other phases, which were identified through X-ray diffraction (XRD) and scanning electronic microscopy (SEM). Conversely, the addition of ZnO did not result in additional phases. These unexpected phases affected directly the bulk conductivity and the activation energy for conduction.













Similar content being viewed by others
Data availability
The data that support the findings of this work are available on request from the corresponding author.
References
W. Zhang, Y.H. Hu, Progress in proton-conducting oxides as electrolytes for low-temperature solid oxide fuel cells: from materials to devices. Energy Sci Eng (2021). https://doi.org/10.1002/ese3.886
B. Singh, S. Ghosh, S. Aich, B. Roy, Low temperature solid oxide electrolytes (LT-SOE): a review. J. Power. Sources 339, 103–135 (2017). https://doi.org/10.1016/j.jpowsour.2016.11.019
Y. Zhang, R. Knibbe, J. Sunarso et al., Recent progress on advanced materials for solid-oxide fuel cells operating below 500°C. Adv. Mater. 1700132, 1–33 (2017). https://doi.org/10.1002/adma.201700132
J. Kim, S. Sengodan, S. Kim et al., Proton conducting oxides : a review of materials and applications for renewable energy conversion and storage. Renew. Sustain. Energy Rev. 109, 606–618 (2019). https://doi.org/10.1016/j.rser.2019.04.042
H. Iwahara, T. Esaka, H. Uchida, N. Maeda, Proton conduction in sintered oxides and its application to steam electrolysis for hydrogen production. Solid State Ionics 4, 359–363 (1981)
S. Ricote, N. Bonanos, G. Caboche, Water vapour solubility and conductivity study of the proton conductor BaCe(0.9–x)ZrxY0.1O(3–δ). Solid State Ionics 180, 990–997 (2009). https://doi.org/10.1016/j.ssi.2009.03.016
L. Malavasi, C.A.J. Fisher, M.S. Islam, Oxide-ion and proton conducting electrolyte materials for clean energy applications: structural and mechanistic features. Chem. Soc. Rev. 39, 4370–4387 (2010). https://doi.org/10.1039/b915141a
I.T. Bello, S. Zhai, Q. He et al., Materials development and prospective for protonic ceramic fuel cells. Int. J. Energy Res. 46, 2212–2240 (2022). https://doi.org/10.1002/er.7371
F.J.A. Loureiro, N. Nasani, G.S. Reddy et al., A review on sintering technology of proton conducting BaCeO3-BaZrO3 perovskite oxide materials for protonic ceramic fuel cells. J. Power. Sources 438, 226991 (2019). https://doi.org/10.1016/j.jpowsour.2019.226991
U. Aarthi, K.S. Babu, Grain boundary space charge modulation in BaZr0.8Y0.2-xMxO3−δ with transition metal (M= Ni Co, Fe, and Zn) co-doping. Int. J. Hydrogen Energy 45, 29356–29366 (2020). https://doi.org/10.1016/j.ijhydene.2020.07.207
H.S. Soares, I. Antunes, F.J.A. Loureiro et al., Effect of the addition mechanism of ZnO sintering aid on densification, microstructure and electrical properties of Ba(Zr, Y)O3-δ proton-conducting perovskite. Int. J. Hydrogen Energy 46, 26466–26477 (2021). https://doi.org/10.1016/j.ijhydene.2021.05.109
N. Nasani, Z. Shakel, F.J.A. Loureiro et al., Exploring the impact of sintering additives on the densification and conductivity of BaCe0.3Zr0.55Y0.15O3-δ electrolyte for protonic ceramic fuel cells. J. Alloys Compd. 862, 158640 (2021). https://doi.org/10.1016/j.jallcom.2021.158640
H.W. Kim, J. Seo, J.H. Yu et al., Effect of cerium on yttrium-doped barium zirconate with a ZnO sintering aid: grain and grain boundary protonic conduction. Ceram. Int. 47, 32720–32726 (2021). https://doi.org/10.1016/j.ceramint.2021.08.168
S. Likhittaphon, T. Pukkrueapun, P. Seeharaj et al., Effect of sintering additives on barium cerate based solid oxide electrolysis cell for syngas production from carbon dioxide and steam. Fuel Process. Technol. 173, 119–125 (2018). https://doi.org/10.1016/j.fuproc.2018.01.019
Y. Huang, R. Merkle, J. Maier, Effects of NiO addition on sintering and proton uptake of Ba(Zr, Ce, Y)O 3−δ. J Mater Chem A (2021). https://doi.org/10.1039/d1ta02555d
B. Wang, L. Bi, X.S. Zhao, Exploring the role of NiO as a sintering aid in BaZr0,1Ce0,7Y0,2O3-ẟ electrolyte for proton-conducting solid oxide fuel cells. J. Power. Sources 399, 207–214 (2018). https://doi.org/10.1016/j.jpowsour.2018.07.087
Z.U.D. Babar, M.B. Hanif, J.T. Gao et al., Sintering behavior of BaCe0.7Zr01.Y0.2O3-δ electrolyte at 1150 °C with the utilization of CuO and Bi2O3 as sintering aids and its electrical performance. Int. J. Hydrogen Energy (2021). https://doi.org/10.1016/j.ijhydene.2021.12.075
L. Gui, Y. Ling, G. Li et al., Enhanced sinterability and conductivity of BaZr0.3Ce0.5Y0.2O3−δ by addition of bismuth oxide for proton conducting solid oxide fuel cells. J. Power. Sources 301, 369–375 (2016). https://doi.org/10.1016/j.jpowsour.2015.10.029
P. Babilo, S.M. Haile, Enhanced sintering of yttrium-doped barium zirconate by addition of ZnO. J. Am. Ceram. Soc. 88, 2362–2368 (2005). https://doi.org/10.1111/j.1551-2916.2005.00449.x
S. Tao, J.T.S. Irvine, Conductivity studies of dense yttrium-doped BaZrO3 sintered at 1325°C. J. Solid State Chem. 180, 3493–3503 (2007). https://doi.org/10.1016/j.jssc.2007.09.027
M. Amsif, D. Marrero-López, J.C. Ruiz-Morales et al., The effect of Zn addition on the structure and transport properties of BaCe0.9−xZrxY0.1O3−δ. J. Eur. Ceram. Soc. 34, 1553–1562 (2014). https://doi.org/10.1016/j.jeurceramsoc.2013.12.008
Y. Zhang, Y. Yao, J. Ren et al., MnO2 as an effective sintering aid for difficult-to-sinter LiTaO3-based ceramics: densification and dielectric properties. J. Alloys Compd. (2020). https://doi.org/10.1016/j.jallcom.2020.154546
T.S. Zhang, J. Ma, L.B. Kong et al., Iron oxide as an effective sintering aid and a grain boundary scavenger for ceria-based electrolytes. Solid State Ionics 167, 203–207 (2004). https://doi.org/10.1016/j.ssi.2004.01.006
M. Hakim, J.H. Joo, C.Y. Yoo et al., Enhanced chemical stability and sinterability of refined proton-conducting perovskite: Case study of BaCe0.5Zr0.3Y0.2O3-δ. J. Eur. Ceram. Soc. 35, 1855–1863 (2015). https://doi.org/10.1016/j.jeurceramsoc.2014.11.033
L. Ge, J. Jiao, Z. Zhu et al., A facile method to fabricate proton-conducting BaZr0·85Y0·15O3-δ electrolyte with a large grain size and high conductivity. Ceram. Int. 45, 24946–24952 (2019). https://doi.org/10.1016/j.ceramint.2019.08.202
R.R. Chien, C.S. Tu, V.H. Schmidt et al., Synthesis and characterization of proton-conducting Ba(Zr0.8-XCexY0.2)O2.9 ceramics. Solid State Ionics 181, 1251–1257 (2010). https://doi.org/10.1016/j.ssi.2010.07.024
N. Nasani, P.A.N. Dias, J.A. Saraiva, D.P. Fagg, Synthesis and conductivity of Ba(Ce, Zr, Y)O3-δ electrolytes for PCFCs by new nitrate-free combustion method. Int. J. Hydrogen Energy 38, 8461–8470 (2013). https://doi.org/10.1016/j.ijhydene.2013.04.078
Y. Goto, T. Takada, Phase diagram of the system BaO-Fe2O3. J. Am. Ceram. Soc. 43, 150–153 (1960). https://doi.org/10.1111/j.1151-2916.1960.tb14330.x
M. Balaguer, Y.J. Sohn, D. Kobertz et al., Characterization of Y and Mn co-substituted BaZrO3 ceramics: material properties as a function of the substituent concentration. Solid State Ionics 382, 115959 (2022). https://doi.org/10.1016/j.ssi.2022.115959
C. Zhang, H. Zhao, N. Xu et al., Influence of ZnO addition on the properties of high temperature proton conductor Ba1.03Ce0.5Zr0.4Y0.1O3−δ synthesized via citrate–nitrate method. Int. J. Hydrogen Energy 34, 2739–2746 (2009). https://doi.org/10.1016/j.ijhydene.2009.01.061
L.S. Hagy, K. Ramos, M.V. Gelfuso et al., Effects of ZnO addition and microwave sintering on the properties of BaCe0.2Zr0.7Y0.1O3-δ proton conductor electrolyte. Ceram. Int. 49, 17261–17270 (2023). https://doi.org/10.1016/j.ceramint.2023.02.092
G.S. Reddy, R. Bauri, A novel route to enhance the sinterability and its effect on microstructure, conductivity and chemical stability of BaCe0.4Zr0.4Y0.2O3-δ proton conductors. Mater. Chem. Phys. 216, 250–259 (2018). https://doi.org/10.1016/j.matchemphys.2018.05.023
J.H. Lee, S.M. Choi, J.H. Lee et al., Effect of sintering atmosphere on phase stability, and electrical conductivity of proton-conducting Ba(Zr0.84Y0.15Cu 0.01)O3-δ. Int. J. Hydrogen Energy 39, 7100–7108 (2014). https://doi.org/10.1016/j.ijhydene.2014.02.072
A. Løken, T.S. Bjørheim, R. Haugsrud, The pivotal role of the dopant choice on the thermodynamics of hydration and associations in proton conducting BaCe0.9X0.1O3−δ (X = Sc, Ga, Y, In, Gd and Er). J Mater Chem A 3, 23289–23298 (2015). https://doi.org/10.1039/C5TA04932F
Á. Triviño-Peláez, D. Pérez-Coll, J. Mosa et al., Enhanced proton conductivity and stability of Ba-deficient BaCe0.8Y0.2O3-δ. J. Power. Sources 493, 229691 (2021). https://doi.org/10.1016/j.jpowsour.2021.229691
S. Nikodemski, J. Tong, R. O’Hayre, Solid-state reactive sintering mechanism for proton conducting ceramics. Solid State Ionics 253, 201–210 (2013). https://doi.org/10.1016/j.ssi.2013.09.025
L.P. Wendler, K. Ramos, D.M.P.F. Souza, Investigation about the reason of limited grain growth of Y-doped barium zirconate. Ceram. Int. 45, 19120–19126 (2019). https://doi.org/10.1016/j.ceramint.2019.06.158
T. Negas, R.S. Roth, Phase equilibria and structural relations in the system BaMnO3-x. J. Solid State Chem. 3, 323–339 (1971). https://doi.org/10.1016/0022-4596(71)90068-5
F.J.A. Loureiro, D. Pérez-Coll, V.C.D. Graça et al., Proton conductivity in yttrium-doped barium cerate under nominally dry reducing conditions for application in chemical synthesis. J Mater Chem A 7, 18135–18142 (2019). https://doi.org/10.1039/c9ta04584h
H. Wang, R. Peng, X. Wu et al., Sintering behavior and conductivity study of yttrium-doped BaCeO3-BaZrO3 solid solutions using ZnO additives. J. Am. Ceram. Soc. 92, 2623–2629 (2009). https://doi.org/10.1111/j.1551-2916.2009.03204.x
S.M. Haile, D.L. West, J. Campbell, The role of microstructure and processing on the proton conducting properties of gadolinium-doped barium cerate. J. Mater. Res. 13, 1576–1595 (1998). https://doi.org/10.1557/JMR.1998.0219
D. Gao, R. Guo, Structural and electrochemical properties of yttrium-doped barium zirconate by addition of CuO. J. Alloys Compd. 493, 288–293 (2010). https://doi.org/10.1016/j.jallcom.2009.12.082
H. Sun, X. Guo, J. Li et al., Effect of grain size on the electrical performance of BaZr0.1Ce0.7Y0.1Yb0.1O3-ẟ solid electrolytes with addition of NiO. Ceram. Int. 45, 622–626 (2019). https://doi.org/10.1016/j.ceramint.2018.09.217
J. Wallis, L. Urban, C. Grimmer et al., Structural and electrical properties of BaZr0.7Ce0.2Y0.1O3−δ proton conducting ceramic fabricated by spark plasma sintering. Solid State Ionics 345, 115118 (2020). https://doi.org/10.1016/j.ssi.2019.115118
M. Amsif, D. Marrero-López, J.C. Ruiz-Morales et al., Effect of sintering aids on the conductivity of BaCe0.9Ln0.1O3−δ. J. Power. Sources 196, 9154–9163 (2011). https://doi.org/10.1016/j.jpowsour.2011.06.086
A.K. Baral, Reduction in sintering temperature of stable proton conductor BaCe0.35Zr0.5Y0.15O3-δ prepared by sol–gel method and its transport properties. Solid State Ionics 272, 107–111 (2015). https://doi.org/10.1016/j.ssi.2015.01.005
Funding
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001; CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico Finance Code: 406915/2021-0; Fundação Araucária de Apoio ao Desenvolvimento Científico e Tecnológico do Estado do Paraná.
Author information
Authors and Affiliations
Contributions
FNV: Conceptualization, Methodology, Validation, Formal Analysis, Investigation, Resources, Data Curation, Writing—original draft. KR: Formal Analysis, Investigation, Validation, Writing—review & editing. ALC: Conceptualization, Funding Acquisition, Supervision, Project Administration. ASAC: Conceptualization, Funding Acquisition, Supervision, Project Administration.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Viechineski, F.N., Ramos, K., Chinelatto, A.L. et al. Optimizing the densification of BaCe0.2Zr0.7Y0.1O3-δ proton conducting electrolyte using Fe2O3, Mn2O3 and ZnO sintering aids. J Mater Sci: Mater Electron 34, 2165 (2023). https://doi.org/10.1007/s10854-023-11560-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-11560-x