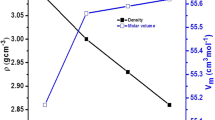
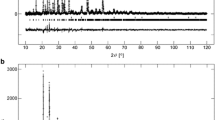
Abstract
In the present study, lithium doped vanadium pentoxide LixV2O5 (0.2 ≤ x ≤ 0.8) specimens have been prepared by the solid-state synthesis method, which possesses an ionic and electronic conducting nature. Lithium incorporates vanadium pentoxide to increase electrical conductivity as well as to enhance energy storage capacity. The undoped V2O5 samples show orthorhombic structures, which are slightly tilted towards monoclinic due to lithium doping. These structural and compositional studies have been carried out using X-ray diffraction spectra and X-ray photoelectron spectroscopy. The stretching and vibrational modes of prepared samples are obtained by Fourier Transform Infrared Spectrometer. The significant shifting of optical absorption is observed and hence the optical energy band decreases from 3.37 to 2.44 eV for the present specimens. Electrochemical analysis in the potential window of −0.5 to 0.5 V (scan rate of 5 mV/s) with 2 M KCl electrolyte solution is conducted to get maximum specific capacitance of 529 Fg−1 for Li0.2V1.8O4.6 specimen. The first and fifth cycles of Cyclic Voltammetry suggest that the charging and discharging of LixV2O5 are in accordance with doped component proportion. The dielectric parameters have been recorded with a frequency response of 10 Hz to 1 MHz. The ac conductivity data has been interpreted by Jonscher’s power law.














Similar content being viewed by others
Data availability
Data will be made available on reasonable request.
References
Y. Sun, Z. Xie, Y. Li, RSC Adv. 8, 39371 (2018). https://doi.org/10.1039/c8ra07326k
S.H. Chun, H.Y. Kim, H. Jang, Y. Lee, A. Jo, N.S. Lee, H.K. Yu, Y. Lee, M.H. Kim, C. Lee, CrystEngComm 19, 3455 (2017). https://doi.org/10.1039/c7ce00637c
C.K. Christensen, D.R. Sørensen, J. Hvam, D.B. Ravnsbæk, Chem. Mater. 31, 512 (2019)
X. Huang, X. Rui, H.H. Hng, Q. Yan, Part. Part. Syst. Charact. 32, 276 (2015)
A. Langar, N. Sdiri, H. Elhouichet, M. Ferid, Eur. Phys. J. Plus (2016). https://doi.org/10.1140/epjp/i2016-16421-y
L. Mai, Q. An, Q. Wei, J. Fei, P. Zhang, X. Xu, Y. Zhao, M. Yan, W. Wen, L. Xu, Small 10, 3032 (2014). https://doi.org/10.1002/smll.201302991
S. Kumar, S.D. Bukkitgar, S. Singh, Pratibha, V. Singh, K.R. Reddy, N.P. Shetti, C.V. Reddy, V. Sadhu, S. Naveen, ChemistrySelect 4, 5322 (2019)
A. Sakunthala, M.V. Reddy, S. Selvasekarapandian, B.V.R. Chowdari, P.C. Selvin, Energy Environ. Sci. 4, 1712 (2011)
T.M. Tolhurst, B. Leedahl, J.L. Andrews, S. Banerjee, A. Moewes, J. Mater. Chem. A 5, 23694 (2017)
A. Dey, M.K. Nayak, A.C.M. Esther, M.S. Pradeepkumar, D. Porwal, A.K. Gupta, P. Bera, H.C. Barshilia, A.K. Mukhopadhyay, A.K. Pandey, K. Khan, M. Bhattacharya, D.R. Kumar, N. Sridhara, A.K. Sharma, Sci. Rep. 6, 1 (2016). https://doi.org/10.1038/srep36811
M. Liu, B. Su, Y. Tang, X. Jiang, A. Yu, Adv. Energy Mater. 7, 1 (2017). https://doi.org/10.1002/aenm.201700885
Y. Lu, L. Liu, D. Mandler, P.S. Lee, J. Mater. Chem. C 1, 7380 (2013)
X. Liu, J. Zeng, H. Yang, K. Zhou, D. Pan, RSC Adv. 8, 4014 (2018). https://doi.org/10.1039/c7ra12523b
W. Zhong, J. Huang, S. Liang, J. Liu, Y. Li, G. Cai, Y. Jiang, J. Liu, ACS Energy Lett. 5, 31 (2020)
I. Mjejri, A. Rougier, M. Gaudon, Inorg. Chem. 56, 1734 (2017)
J.N. Spencer, A. Folli, H. Ren, D.M. Murphy, J. Mater. Chem. A 9, 16917 (2021). https://doi.org/10.1039/d1ta02352g
M. Yuan, Y. Li, Q. Chen, C. Chen, X. Liu, W. Zeng, R. Wang, S. Xiao, Electrochim. Acta 323, 134822 (2019)
D. McNulty, D.N. Buckley, C. O’Dwyer, J. Power. Sources 267, 831 (2014)
X. Zhang, X. Sun, X. Li, X. Hu, S. Cai, C. Zheng, J. Energy Chem. 59, 343 (2021). https://doi.org/10.1016/j.jechem.2020.11.022
Z. Zhang, G. Yao, X. Zhang, J. Ma, H. Lin, Ceram. Int. 41, 4523 (2015)
K.P. Thummer, A.R. Tanna, H.H. Joshi, A.I.P. Conf, Proc. 1837, 1 (2017)
A.R. Tanna, S.S. Srinivasan, H.H. Joshi, J. Mater. Sci. Mater. Electron. 31, 9306 (2020)
V. Balasubramani, J. Chandrasekaran, T.D. Nguyen, S. Maruthamuthu, R. Marnadu, P. Vivek, S. Sugarthi, Sens. Actuators A Phys. 315, 112333 (2020)
B. Hu, L. Li, X. Xiong, L. Liu, C. Huang, D. Yu, C. Chen, J. Solid State Electrochem. 23, 1315 (2019). https://doi.org/10.1007/s10008-019-04220-w
V. Mounasamy, G. Srividhya, N. Ponpandian, Energy Adv (2023). https://doi.org/10.1039/d3ya00100h
X. Li, Y. Su, X. Lang, L. Li, C. Yao, K. Cai, Ionics (Kiel). 28, 1511 (2022)
B. Bera, P. Sekhar Das, M. Bhattacharya, S. Ghosh, A.K. Mukhopadhyay, A. Dey, J. Phys. D Appl. Phys. 49, 085303 (2016)
M.F.G. Huila, H.E. Toma, Electrochim. Acta 278, 236 (2018)
J. Huang, H. Liu, T. Hu, Y.S. Meng, J. Luo, J. Power. Sources 375, 21 (2018)
A. Caballero, M. Cruz, L. Hernán, M. Melero, J. Morales, E.R. Castellón, J. Electrochem. Soc. 152, A552 (2005)
G. Bodurov, T. Ivanova, K. Gesheva, Phys. Procedia 46, 149 (2013)
Y. Chai, F.Y. Ha, F.K. Yam, Z. Hassan, Procedia Chem. 19, 113 (2016). https://doi.org/10.1016/j.proche.2016.03.123
T. Ivanova, A. Harizanova, T. Koutzarova, N. Krins, B. Vertruyen, Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 165, 212 (2009). https://doi.org/10.1016/j.mseb.2009.07.013
S.D. Rezaei, S. Shannigrahi, S. Ramakrishna, Sol. Energy Mater. Sol. Cells 159, 26 (2017)
B.T. Rao, S. Cole, Rasayan J. Chem. 12, 1557 (2019)
Y. Feng, S. Lin, S. Huang, S. Shrestha, G. Conibeer, Y. Feng, S. Lin, S. Huang, S. Shrestha, G. Conibeer, J. Appl. Phys. 117, 125701 (2015). https://doi.org/10.1063/1.4916090
P. Jani, H. Desai, B.S. Madhukar, A. Tanna, Mater. Res. Innov. 26, 189 (2022)
M. Rafique, M. Hamza, M.B. Tahir, S. Muhammad, A.G. Al-Sehemi, J. Mater. Sci. Mater. Electron. 31, 12913 (2020)
H. Shen, I.R. Ie, C.S. Yuan, C.H. Hung, W.H. Chen, Environ. Sci. Pollut. Res. 23, 5839 (2016)
M.N. Siddique, A. Ahmed, T. Ali, P. Tripathi, AIP Conf. Proc. (2018). https://doi.org/10.1063/1.5032362
K. Schneider, J. Mater. Sci. Mater. Electron. 31, 10478 (2020)
R. Vijaya Kumar, Y. Diamant, A. Gedanken, Chem. Mater. 12, 2301 (2000)
S.T. Akinkuade, W.E. Meyer, J.M. Nel, Physica B Condens. Matter 575, 411694 (2019). https://doi.org/10.1016/j.physb.2019.411694
J. Zhou, Y. Gao, Z. Zhang, H. Luo, C. Cao, Z. Chen, L. Dai, X. Liu, Sci. Rep. 3, 1 (2013). https://doi.org/10.1038/srep03029
S. Khan, K. Singh, Sci. Rep. 10, 1 (2020). https://doi.org/10.1038/s41598-020-57836-8
A. Sawaby, M.S. Selim, S.Y. Marzouk, M.A. Mostafa, A. Hosny, Physica B Condens. Matter 405, 3412 (2010). https://doi.org/10.1016/j.physb.2010.05.015
S. Kim, M. Taya, C. Xu, J. Electrochem. Soc. 156, 40 (2009)
N. Guru Prakash, M. Dhananjaya, B. Purusottam Reddy, K. Sivajee Ganesh, A. Lakshmi Narayana, O.M. Hussain, Mater. Today Proc. 3, 4076 (2016)
K.Y. Pan, D.H. Wei, Nanomaterials 6, 140 (2016)
G. Wee, H.Z. Soh, Y.L. Cheah, S.G. Mhaisalkar, M. Srinivasan, J. Mater. Chem. 20, 6720 (2010)
Y. Zhang, J. Zheng, Q. Wang, T. Hu, C. Meng, RSC Adv. 6, 93741 (2016). https://doi.org/10.1039/c6ra16262b
J. Zheng, Y. Zhang, X. Jing, Q. Wang, T. Hu, N. Xing, C. Meng, Mater. Chem. Phys. 186, 5 (2017)
D. Majumdar, M. Mandal, S.K. Bhattacharya, ChemElectroChem 6, 1623 (2019)
Z.J. Lao, K. Konstantinov, Y. Tournaire, S.H. Ng, G.X. Wang, H.K. Liu, J. Power. Sources 162, 1451 (2006)
H.M. El-Mallah, Acta Phys. Pol. A 122, 174 (2012)
I.S. Elashmawi, N.S. Alatawi, N.H. Elsayed, Results Phys. 7, 636 (2017)
N.A. Hegab, H.M. El-mallah, Acta Phys. Pol. A 116, 1048 (2009)
Acknowledgements
MAB is thankful to Dr. G. M. Sutariya, Sir P. P. Institute of Science, Bhavnagar for providing lab facilities and technical support.
Funding
The authors declare that there are no funds, grants, or other support are available during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors have participated in the conception and design, analysis, and interpretation of the data. The drafting of the article or revising is done by all authors. A final manuscript was read and approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
We declare the authors of the article “Effect of Li-ion doping on structural, optical and electrochemical properties of V2O5” have no conflict of interest. There is no financial support available for this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhatt, M.A., Tanna, A.R. Effect of Li-ion doping on structural, optical and electrochemical properties of V2O5. J Mater Sci: Mater Electron 34, 2146 (2023). https://doi.org/10.1007/s10854-023-11462-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-11462-y