Abstract
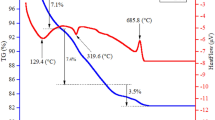
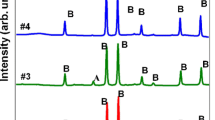
In this study, holmium orthoferrite (o-HoFeO3) nanoparticles were successfully synthesized by simple co-precipitation method without adding gelling organic polymers. Structures, morphologies, elemental composition, thermal, and magnetic properties of the product were characterized by powder X-ray diffraction (PXRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy dispersive X-ray spectroscopy (EDX), thermogravimetry and differential scanning calorimetry (TG-DSC), and vibrating sample magnetometer (VSM). After annealing the precursors at different temperatures for 60 min, nanocrystrals with orthorhombic perovskite structure were obtained. Crystallite size (DPXRD = 22.13–53.74 nm), particle size (DTEM/SEM = 20–60 nm), and lattice volume (V = 223.65–224.99 Å3) increased with the annealing temperature. The optimal annealing temperature for obtaining the single crystalline phase of o-HoFeO3 was ≥ 750 °C, and the o-HoFeO3 crystalline phase remained stable at temperatures ≥ 1050 °C. The synthesized o-HoFeO3 nanoparticles exhibited a uniform spherical shape, with a size of 20–60 nm, and exhibited the properties of a paramagnetic material at 300 K. Notably, the coercive force and residual magnetism of the synthesized material were much smaller than those reported in previous studies for similar materials. The experimental results in this work may provide fundamental support to the research and development of magnetic material.








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
N. Singh, J.Y. Rhee, S. Auluck, Electronic and magneto-optical properties of rare-earth orthoferrite RFeO3 (R = Y, Sm, Eu, Gd and Lu). J. Korean Phys. Soc. 53, 806–816 (2010). https://doi.org/10.3938/jkps.53.806
J. Ding, X. Lu, H. Shu, J. Xie, H. Zhang, Microwave-assisted synthesis of perovskite ReFeO3 (Re: La, Sm, Su, Gd) photocatalyst. Mater. Sci. Eng. B 171, 31–34 (2010). https://doi.org/10.1016/j.mseb.2010.03.050
T.V. Manh, Y. Pham, T.L. Phan, N.T. Dang, N. Tran, H.R. Park, B.W. Lee, Electronic structure and magnetocaloric effect of Sr-doped SmCoO3 perovskite. J. Electron. Mater. 5, 177–187 (2023). https://doi.org/10.1007/s11664-022-09943-7
S.K. Sahu, S. Tanasescu, B. Scherrer, C. Marinnescu, A. Navrotsky, Energetics of lanthanide cobalt perovskites: LnCoO3−δ (Ln = La, Nd, Sm, Gd). J. Mater. Chem. A 38, 19490–19496 (2015). https://doi.org/10.1039/C5TA03655K
B.K. Ostafiychuk, H.M. Kolkovska, I.P. Yaremily, B.I. Tachiy, P.I. Kolkovskyi, N.Y. Ivanichok, S.I. Yaremiy, Synthesis and electrochemical properties of LnMnO3 perovskite nanoparticles. Phys. Chem. Solid State 21, 219–226 (2020). https://doi.org/10.15330/pcss.21.2.219-226
M. Escote, A.M.L. da Silva, J. Matos, R.F. Jardim, General properties of polycrystalline LnNiO3 (Ln = Pr, Nd, Sm) compounds prepared through different precursors. J. Solid State Chem. 151, 298–307 (2000). https://doi.org/10.1006/jssc.2000.8657
Z. Yu, Y. Sun, W. Wei, L. Lu, X. Wang, Preparation of NdCrO3 nanoparticles and their catalytic activity in the thermal decomposition of ammonium perchlorate by DSC/TG-MS. J. Therm. Anal. Cal. 97, 903–909 (2000). https://doi.org/10.1007/s10973-009-0091-7
A.T. Nguyen, T.A. Nguyen, V.O. Mittova, H.D. Ngo, M.L.P. Le, D.Q. Nguyen, N.V.H. MittovaIYa, S. Hiroshi, H.T. Bui, T.L. Nguyen, Facile co-precipitation synthesis of NdFeO3 perovskite nanoparticles for lithium-ion battery anodes. J. Mater. Sci. Mater. Electron. 33, 19082–19092 (2022). https://doi.org/10.1007/s10854-022-08745-1
L.C.P. Sonia, S.A. João, W. Alain, D. Mathieu, M.H. Delville, C.F.G.C. Geraldes, Synthesis and characterization of rare earth orthoferrite LnFeO3 nanoparticles for biomaging. Eur. J. Inorg. Chem. 31, 3570–3578 (2018). https://doi.org/10.1002/ejic.201800468
A.T. Nguyen, W.G. Kidanu, V.O. Mittova, V.H. Nguyen, D.Q. Nguyen, M.L.P. Le, Mittova IYa, Kim IIT, Nguyen TL, Tailored HoFeO3–Ho2O3 hybrid perovskite nanocomposite as stable anode material for advanced lithium-ion storage. Inter. J. Ener. Res. 46, 2051–2063 (2021). https://doi.org/10.1002/er.7273
K.O. Ogunniran, G. Murugadoss, R. Thangamuthu, P. Periasamy, Evaluation of nanostructured Nd0.7Co0.3FeO3 perovskite obtanied via hydrothermal method as anode mateiral for Li-ion battery. Mater. Chem. Phys. 248, 122944 (2020). https://doi.org/10.1016/j.matchemphys.2020.122944
P. Tang, X. Xie, H. Chen, C. Lv, Y. Ding, Synthesis of nanoparticlate PrFeO3 by sol-gel method and its visible-light photocatalytic activity. Ferroelectrics 546, 181–187 (2019). https://doi.org/10.1080/00150193.2019.1592470
C.O. Deniz, T. Ahmet, C. Erdal, Synthesis and characterizations of LaMnO3 perovskite powders uisng sol-gel method. J. Mater. Sci. Mater. Electron. 32, 15544–15562 (2021). https://doi.org/10.1007/s10854-021-06104-0
Z. Zhou, L. Guo, H. Yang, Q. Liu, F. Ye, Hydrothermal synthesis and magnetic properties of multiferroic rare-earth orthoferrites. J. Alloys Compd. 583, 21–31 (2014). https://doi.org/10.1016/j.jallcom.2013.08.129
M.K. Mozhgan, M. Noroozifar, M. Yousefi, S. Jahani, Chemical synthesis and characterization of perovskite NdFeO3 nanocrystals via a co-precipitation method. Int. J. Nanosci. Nanotechnol. 9, 7–14 (2013)
M. Yousefi, S.S. Zeid, M.K. Mozhgan, Synthesis and characterization of nano-structured perovskite type neodymium orthoferrite NdFeO3. Curr. Chem. Lett. 6, 23–30 (2017). https://doi.org/10.5267/j.ccl.2016.10.002
M. Nakhael, D.S. Khoshnoud, Structural, magnetic, and electrical properties of RFeO3 (R = Dy, Ho, Yb & Lu) compounds. J. Mater. Sci. Mater. Electron. 32, 14286–14300 (2021). https://doi.org/10.1007/s10854-021-05992-6
Z. Habib, K. Majid, M. Ikram, K. Sultan, Influence of Ni substitution at B-site for Fe3+ ions on morphological, optical, and magnetic properties of HoFeO3 ceramics. Appl. Phys. Mater. Sci. Process 122, 550 (2016). https://doi.org/10.1007/s00339-016-0082-z
T.K.C. Nguyen, A.T. Nguyen, V.O. Mittova, H.D. Chau, T.L. Nguyen, Mittova IYa, Bui XV, Effect of annealing temperature and cadmium doping on structure and magnetic properties of neodymium orthoferrite nanoparticles synthesized by a simple co-precipitation method. Process Appl. Ceram. 16, 321–327 (2022). https://doi.org/10.2298/PAC2204321N
H.D.T. Pham, L.T.T. Nguyen, V.O. Mittova, H.D. Chau, I Ya. Mittova, A.T. Nguyen, X.V. Bui, Structural, optical and magnetic properties of Sr and Ni co-doped YFeO3 nanoparticles prepared by simple co-precipitation method. J. Mater. Sci. Mater. Electron. 33, 14356–14367 (2022). https://doi.org/10.1007/s10854-022-08360-0
T.H.D. Pham, H.D. Chau, A.T. Nguyen, Cd-doped NdFeO3 nanoparticles: synthesis and optical properties study. J. Mater. Sci. Mater. Electron. 33, 3546–3555 (2022). https://doi.org/10.1007/s10854-021-07546-2
T.H.D. Pham, A.T. Nguyen, X.V. Bui, Optical and magnetic characteristics of LaFeO3 nanoparticles synthesized by simple co-precipitation method using ethanol. Asian J. Chem. 34, 1279–1283 (2022). https://doi.org/10.14233/ajchem.2022.23606
A.T. Nguyen, I. Y.A. Mittova, O.V. Almjasheva, S.A. Kirillova, V.V. Gusarov, Influence of the preparation condition on the size and morphology of nanocrystalline lanthanum orthoferrite. Glass Phys. Chem. 34, 756–761 (2008). https://doi.org/10.1134/S1087659608060138
C.E. Housecroft, A.G. Sharpe, Inorganic chemistry, 2nd edn. (Prentice Hall, Pearson, Hoboken, 2005)
P. Caro, M. Lemaitre, M. Blassé, Hydroxycarbonates deterres rares Ln2(CO3)x(OH)2(3–x) nH2O. C.R. Seances. Acad. Sci. Ser. C. 269, 687 (1969)
T.K.C. Nguyen, A.T. Nguyen, X.V. Bui, Optical and magnetic properties of YFeO3 nanoparticles synthesized by a co-precipitation method at high temperature. Chem Papers 76, 923–930 (2022). https://doi.org/10.1007/s11696-021-01913-3
N. Imanaka, Physical and chemical properties of rare earth oxides, binary rare earth oxides (Kluwer Academic Publishers, Dordrecht, 2004)
C. Sasikala, N. Durairaj, I. Baskaran, B. Sathyaseelan, M. Henini, Transition metal titanium (Ti) doped LaFeO3 nanoparticles for enhanced optical and magnetic properties. J Alloys Compd 712, 870–877 (2017). https://doi.org/10.1016/j.jallcom.2017.04.133
A.T. Nguyen, T.T.L. Nguyen, X.V. Bui, T.H.D. Nguyen, D.H. Lieu, T.M.L. Le, V. Pham, Optical and magnetic properties of HoFeO3 nanocrystals prepared by a simple co-precipitation method using ethanol. J. Alloys Compd. 834, 155098 (2020). https://doi.org/10.1016/j.jallcom.2020.155098
Y. Albadi, A.A. Sirotkin, V.G. Semenov, R.S. Abiev, V.I. Popkov, Synthesis of superparamagnetic GdFeO3 nanoparticles using a free impinging-jets microreactor. Rus Chem. Bull. Inter. Ed. 69, 1290–1295 (2020). https://doi.org/10.1007/s11172-020-2900-x
Y. Cao, M. Xiang, W. Zhao, G. Wang, Z. Feng, B. Kang, A. Stroppa, J. Zhang, W. Ren, S. Cao, Magnetic phase transition and giant anisotropic magnetic entrpy change in TbFeO3 single crystal. J. Appl. Phys. 119, 063904 (2016). https://doi.org/10.1063/1.4941105
Y. Moriya, New mechanism of anisotropic superexchange interaction. Phys. Rev. Lett. 4, 228–230 (1960). https://doi.org/10.1103/PhysRevLett.4.228
A. Jaiswal, R. Das, S. Adyanthaya, P. Poddar, Surface effects on morin transition, exchange bias, and enchanced spin reorientation in chemically synthesized DyFeO3 nanoparticles. J. Phys. Chem. C 115, 2954–2960 (2011). https://doi.org/10.1021/jp109313w
M. Johnsson, P. Lemmens, Crystallography and chemistry of perovskites, 1st edn. (Wiley, Hoboken, 2007), pp.1–11
B.D. Cullity, C.D. Graham, Introduction to magnetic materials, 2nd edn. (Wiley, Canada, 2009)
J. Su, X. Lu, C. Zhang, J. Zhang, H. Sun, C. Ju, Z. Wang, K. Min, F. Huang, J. Zhu, Study on dielectric and magnetic properties of Ho3Fe5O12 ceramics. Phys. B 407, 485–488 (2012). https://doi.org/10.1016/j.physb.2011.11.020
O. Opuchovic, A. Beganskiene, A. Karreiva, Sol-gel derived Tb3Fe5O12 and Y3Fe5O12 garnets: synthesis, phase purity, micro-structure and improved design of morphology. J. Alloys Compd. 647, 189–197 (2015). https://doi.org/10.1016/j.jallcom.2015.05.169
Funding
The authors would like to thank Sai Gon University, Vietnam for the financial support, through Grant No. CSA 2022-12.
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, and formal analysis, Nguyen AT, Le TTT, Tran GTL, and Tomina EV; validation, Bui XV, Vo QM, and Le HP; writing–original draft preparation, Nguyen AT, Bui XV, and Le TTT; writing–review and editing, Bui XV, Tomina EV, and Nguyen AT. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors maintain that they have no conflict of interest to be described in this communication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thuy, L.T.T., Loan, T.G.T., Tomina, E.V. et al. Structural, thermal and magnetic properties of orthoferrite holmium nanoparticles synthesized by a simple co-precipitation method. J Mater Sci: Mater Electron 34, 1499 (2023). https://doi.org/10.1007/s10854-023-10923-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-10923-8