Abstract
The oxide glass–ceramics is a promising class of solid state materials because they are using thermally stable and chemically inert glass oxide matrices. Development of such efficient glass matrices suitable for creation of glass–ceramic materials for several purposes is an important practical task. The xLi2O–yV2O5–(100 − x − y)B2O3 undoped glass and 47Li2O–2V2O5–50B2O3–1La0.3Eu0.7VO4 glass samples with crystalline nanoinclusions were synthesized and investigated using XRD, IR and UV–Vis spectroscopy and UV band-to-band excitation of luminescence. The synthesized glass samples are characterized by wide band photoluminescence emission with maximum at 570 nm and intensity increased with increase of Li2O concentration. The excitation spectra consist of three bands with maxima located at 270, 320 and 365 nm. The observed concentration dependencies of spectral distributions in the absorption and excitation spectra are explained by influence of the lithium ions on a ratio between triborate and tetraborate groups in the glass networks. The assumption is made that the observed wide band photoluminescence emission of the glass matrix can appear as a result of recombination processes between the defects in borate networks and the broken vanadate groups. The crystalline component in the doped glass samples is found to not affect the luminescence properties of the glass matrix. Intensity of narrow band photoluminescence emission of the crystalline component is up to 10 times more intense than that of the glass matrix wide band emission. The synthesized type of the glass matrices has promising characteristics for the use of developed materials in lighting devices, as it allows improving the spectral distribution of light emission towards the white light.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Oxide–based glass ceramics is a promising class of solid state materials because they use thermally stable and chemically inert glass oxide matrix that can be filled with oxide micro and nanosized crystalline particles distributed through the matrix [1,2,3]. The glass–ceramics are used in various optical applications for creation of new generation of optical fibers, for efficient incoherent and coherent sources of light and for light converting materials [4,5,6,7]. Development of such efficient glass matrices suitable for creation of glass–ceramic materials for several purposes is an important practical task. In many earlier studies, one of the components of the developed glass–ceramics is boron oxide, B2O3, because it is one of the best glass-forming oxides. Diboron trioxide as a glass former is characterized by a wide glass-formation range of concentrations, high transparency, and, moreover, high thermal stability [8,9,10]. Borate-based glass materials possess high mechanical, chemical and thermal stability and they are widely used for the needs of optical science, related techniques and biomedical application. That is why, in our research of glass–ceramic luminescent converters for WLEDs we have focused on the borate glass matrix. Besides, the addition of some network modifiers to boron oxide, usually alkali oxides, can lead to an increase of coordination number of some of the boron atoms from 3 to 4, changing the properties of borate glass. This phenomenon, known as the “borate anomaly” can improve mechanical properties of the glass [10,11,12]. In the present study we use lithium oxide as the second glass former component of the developed ceramics.
The third component of the here developed glass matrices for glass–ceramic composites, vanadium pentoxide V2O5, is favorable for several reasons. Firstly, it is a glass-forming component that modifies the local structure of the borate glass network [13,14,15]. Secondly, V2O5 component increases light absorption of glass matrix from near UV and violet visible ranges. Noted that, in order to keep high transparency of glass and ceramics, the content of the V2O5 component should be enough small and by several estimations it should not exceed 3–5% [16]. Thirdly, it is possible to use vanadate nanoparticles for creation of ceramic compositions, and presence of vanadate groups in both the glass matrix and the nanocrystalline component can significantly increase the efficiency of excitation energy transfer between glass and crystal components in ceramics and to enhance in such a way the intensity of their luminescence [17].
Additional advantage of new glass–ceramic materials developed for the above-described purposes is the ability of glass matrix to luminescence. Recently, we have reported on lithium-vanadate-borate glass, which is characterized by own luminescence at visible light excitation [18]. The observed luminescence emission of the undoped lithium-vanadate-borate glass has been reported for the first time and its mechanisms are not investigated yet. At the same time, understanding of processes responsible for luminescence excitation and emission transitions is very important if the glass composition is used as matrix for creations of ceramic materials, to predict and study possible interaction of glass and crystal phases and excitation energy transfer between them. In order to investigate the origin and nature of the glass matrix emission and its interaction with crystalline activators, in this paper we study the spectral properties of the xLi2O–yV2O5–(100 − x − y)B2O3 glass samples using additionally IR and UV–Vis spectroscopy and UV band-to-band excitation of luminescence.
2 Experimental
A series of the lithium-vanadate-borate undoped glass samples of xLi2O–yV2O5–(100 − x − y)B2O3 composition was synthesized using the melt quenching procedure [19,20,21]. The chemically pure reagents of boric acid H3BO3, lithium carbonate Li2CO3, and vanadium pentoxide V2O5 were taken for the synthesis. The reagents were ground, mixed and placed in porcelain crucibles, then melted in air for 2 h at 400 C and 4 h at 900 C in electric muffle furnace. After melting, the samples were quickly quenched using non-magnet metal plates. The concentration of the V2O5 component was fixed as 2 mol% satisfying the requirements of glass transparency [16]. Thus, for all the samples studied in this paper y = 2 mol%. The concentration x of the Li2O component was varied between 18 and 58 mol% with step of 10 mol%. The doped samples were synthesized by adding the La0.3Eu0.7VO4 crystalline nanoparticles to the initial blend. The concentration of the crystalline component was fixed as 1 mol%.
Verification of the samples amorphicity was performed using a Philips X’Pert Pro Alpha1 diffractometer. IR absorption spectra were measured with a Cary-4000 FTIR spectrometer. UV–Vis absorption spectra were measured with a Varian Cary-5000 spectrometer. Luminescence properties were studied using a Horiba Fluorolog-3 spectrofluorometer.
3 Results
3.1 Phase analysis
The investigation has confirmed that obtained samples are amorphous as indicated by a broad XRD bump located around 2θ = 21–23° and a less intense broad bump around 2θ = 45° (Fig. 1). The intensity of the latter bump is the highest for the 18Li2O–2V2O5–80B2O3 sample, and it decreases gradually with the rise of the Li2O content.
It is noteworthy, that the present diffractograms are in agreement with earlier studies on related materials. Namely, the same broad diffraction bumps around 2θ = 21–25° and 45° were reported before for B2O3–Li2O–MgO, Li2O–Bi2O3–B2O3 and Li2O–B2O3–CuO lithium-borate glasses [22,23,24], whereas some borate glasses (PbO–Li2O–B2O3) are characterized by bumps around 2θ = 29° [25]. The behavior consisting in intensity variations (Fig. 1) correlates with the chemical composition of the samples. It is known that alkali ions in a borate glass induce the change of boron coordination number of boron atoms from three to four and appearance of the non-bridging oxygens [26]. Due to these changes of the covalent bonds in the network structure, various kinds of structural groups are formed such as pentaborate, dipentaborate, diborate, triborate, metaborate, etc., depending on alkali metal applied and its contents [27, 28]. Thus, disappearance of the bump around 2θ = 45° is here attributed to transformations of the three-coordinated borate groups. Taking into account that intensity of the bump around 2θ = 21–25° does not depend on concentration, we should assume that there are at least two types of borate groups forming glass networks in the samples with low Li2O concentrations, and one of them is transformed in presence of the Li2O component. Obviously, this transformation means that the corresponding borate groups are from structural groups including both components (dilithium oxide and diborate trioxide). This assumption will be discussed below together with the analysis of the observed spectral properties.
3.2 IR spectroscopy
As it is already described above, the borate glass has a large number of possible variations of structural units in the borate glass network [29, 30]. Therefore, in order to correctly interpret the luminescence mechanisms in synthesized glass and doped samples, we needed to establish first which structural elements of the borate bonds are dominating. IR spectroscopy methods were used for this purpose. The IR absorption spectra of samples with various concentration ratios of Li2O/B2O3 are shown in Fig. 2. The IR spectra contain four main bands at 480, 690, 990 and 1430 cm−1. The latter two are equally intense for samples with different Li2O concentrations. According to [30, 31] they correspond to the vibrations of the B–O bonds in the BO4 groups (990 cm−1) and BO3 groups (1430 cm−1). The band at 480 cm−1 corresponds to the strain vibrations of the O–B–O bonds in the same BO3 groups. The band at 690 cm−1, which has increased greatly with increasing Li2O content, apparently refers to vibrations of the Li–O bonds. Bands corresponding to vibrations of the borate rings (1300 cm−1) were not detected in the obtained spectra. A weak band related with vibrations of the pyroborate groups (1250 cm−1) was observed for the samples with the highest Li2O content (indicated by an arrow, curve 3 in Fig. 2). Thus, it is established from the IR spectroscopy analysis that the borate network of synthesized glass matrices consists mainly of triborate and tetraborate groups.
3.3 UV–Vis absorption spectroscopy
An increase of the Li2O concentration affects also the color and hue of the glass. The synthesized samples are transparent glass, but they are characterized by the coloration with changing hues from yellow for the samples with high Li2O content to brown for the samples with low Li2O concentration. The absorption spectra revealed that the observed changes of the glass color correlate with smooth gradual monotonous transformations in the light absorption properties of the synthesized glass (Fig. 3).
The absorption spectra consist of the main wide band with maxim positions shifted from 3.85 eV (~ 320 nm) for the sample with x = 18 mol% to 4.1 eV (~ 300 nm) for the sample with x = 58 mol% as well as the long wavelength shoulder near 3.4 eV (365 nm) and a weak band at 2.65 eV (470 nm). The 50% absorption edge is gradually shifted with concentration to the short wavelength range and its position is located at 3.16, 3.25, 3.32, 3.45, 3.6, 3.64 eV for the samples with x = 18, 28, 38, 47, 48, 58 mol%, respectively. A contribution of the 3.4 eV band strongly falls down with the increase of the Li2O concentration, and it practically disappears at 47–48 mol% Li2O content. It is obvious that the observed gradual shift of the main absorption band is caused by the decrease of contribution of the 3.4 eV band to the total spectra with increasing Li2O concentration. Contributions of the 2.65 eV band in the total spectra are very small and do not depend on the Li2O concentration, the observed increase of absorption in the corresponded range for the samples with low Li2O concentrations is caused by contribution of the 3.4 eV band. We can assume here that the 2.65 eV band is related to the V2O5 component. This observation is in line with the published optical characteristics of the vanadium pentoxide [32, 33]. Other absorption bands will be discussed below together with the discussion of luminescence properties.
3.4 Luminescence properties
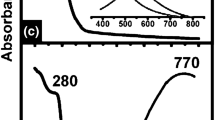
Photoluminescence spectra of the undoped glass samples measured at 280 nm excitations consist of wide unstructured bands in 430–780 nm spectral range. Maximum position of this band is slightly shifted from 580 nm for the sample with the lowest Li2O concentration to 565 nm for the sample with x = 58% (Fig. 4). The spectra measured at 330 nm excitations are observed in the same spectral range and their maxima positions are also slightly shifted towards short wavelengths with increase of the Li2O concentration (Fig. 4, bottom).
For all the applied excitations, intensity of luminescence is distinctively increased with increase of concentration of the Li2O component. Spectra of the La0.3Eu0.7VO4-doped samples contain also narrow lines in the 570–720 nm spectral range which correspond to the well-known emission of the Eu3+ ions [34,35,36]. Peak intensity of the Eu3+ emission is more than 10 times higher as compared with the wide band emission. Noted, that Eu-doping and appearance of the narrow band emission do not affect the intensity of the wide band emission for the spectra measured at 280 nm excitation and slightly decrease the intensity when the emission spectra are measured at 330 nm excitation.
The corresponding photoluminescence excitation spectra of the undoped glass samples are shown in Fig. 5. The spectra contain three bands with maxima positions at 270, 320 and 365 nm. The last band is the most intense for the samples with the lowest Li2O concentration (18 mol%) and quickly falls down with increasing of Li2O content. The increase of the Li2O concentration from 18 to 28 mol% results in a rise of contribution of the 270 and 320 nm bands for about 2 times and the next increase to the 38 mol% leads to rise of contribution of these bands in more than 5 times as compared to the spectra of the sample with the lowest Li2O concentration (18 mol%).
4 Discussion
The obtained results show that concentration dependencies of profiles of the absorption and luminescence excitation spectra exhibit common features and tendencies (Figs. 3 and 5). The most obvious is the behavior of the 365 nm (3.4 eV) band that is present in both absorption and excitation spectra. Intensity of this band strongly falls down with increase of the Li2O content. According to [37], the behavior of this band can be connected with arrangement of the non-bridging oxygens in the borate glass networks. Moreover, vitreous B2O3 forms a glass on cooling, containing mainly BO3 structural units and is thought to comprise locally independent interpenetrating networks made up of boroxol groups and BO3 triangles, as opposed to a large extended single network, and the introduction of alkali cations converts the BO3 triangles into BO4 tetrahedras [38,39,40]. The carried out analysis of fraction of the structural borate groups has shown that at lithium cations concentrations higher than 40 mol%, triborate structural units are not observed in the Raman spectra of the lithium-borate glass [41]. This assumption is partially supported by our results obtained in this work: intensity of the IR absorption peak at 1430 cm−1 ascribed above to the BO4 modes is reduced with increase of the Li2O concentration (Fig. 2). Besides, disappearance of the XRD bump around 2θ = 45° with increase of the Li2O concentration (Fig. 1) that was above attributed (Sect. 3.1) to transformations of the three-coordinated borate groups, now can be clearly connected with conversion of the BO3 triangles into BO4 tetrahedras. Also, if we assign the excitation and absorption bands at 365 nm (3.4 eV) to transitions in the BO3 triangles in the borate glass networks, then we can explain the observed concentration behavior of these bands by conversion of the BO3 triangles into BO4 tetrahedras with increase of the Li2O concentration. Thus, the excitation band at 270 nm, the intensity of which is strongly increased with rise of the Li2O concentration (Fig. 5), should be connected with the BO4 tetrahedras in the borate glass networks. Shift of the main maxima of the absorption spectra in the short wavelength region (Fig. 3) can be a result of increased contribution of absorption of the BO4 groups in the total spectra. The most intense absorption band at 3.85 eV (320 nm) is also observed in the excitation spectra, however, its relative contribution to the excitation spectra is essentially lower than that of the 270 nm band. Despite of this, intensity of the 320 nm excitation band also increases with rise of the Li2O concentration (Fig. 5). It is possible that transitions in diborate groups are responsible for this band.
On the contrary to absorption and excitation spectra, luminescence emission spectra did not demonstrate any spectral dependence with the Li2O concentration (Fig. 5). Only an increase of emission intensity with increase of the Li2O concentration is observed. Different character of the excitation and emission properties leads to a conclusion that the observed luminescence emission should not be ascribed to the transitions to the same local centers. Excitation and emission transitions should occur on different elements of the glass networks. The main point that we must take into account when discuss origin of the emission transitions in the investigated glass matrices and glass–ceramics, that lithium-borate glass without vanadium component is not characterized by such luminescence emission, usually only activator emission is reported for alkali-borate glass [4,5,6, 10, 19]. And vanadate-borate glass has not demonstrated any luminescence emission, too [14,15,16]. Thus, the wideband emission of the lithium-vanadate-borate glass and glass–ceramics is connected with presence of both Li2O and V2O5 components in their composition. The radiation transitions caused by recombination of self-localized holes on bridging oxygen between the (BO4)4− and (BO3)3− groups of the glass matrix and trapped electrons has been reported previously [42]. In the case of our glass compositions, the role of electron traps could be satisfied by broken divanadate pentoxide groups. The structural elements of the V2O5 component can be broken on the VO3−, and VO2+ parts during melting. Taking into account the low content of the V2O5 component in our samples, it is very probable that a lot of these parts will not be combined again together at the quick cooling of the melted solution. The VO2+ groups could be just that electron trap defects in the investigated xLi2O–yV2O5–(100 − x − y)B2O3 glass composition those take part in recombination luminescence processes in the materials under study. Thus, we can make an assumption that the observed wide band photoluminescence emission of the glass matrix can appear as a result of recombination processes between self-localized holes in borate networks and broken vanadate groups.
The above made assumption is supported by spectral properties of the investigated doped glass samples. Presence of the crystalline components does not affect neither the spectral distribution, nor the concentration behavior of both excitation and emission spectra of the glass matrix (Figs. 4, 5). At the same time, the intensity of narrowband photoluminescence emission of the Eu3+ ions in the crystalline component is up to 10 times higher than the intensity of the glass matrix wide band emission (Fig. 4). This evidences the efficient transfer of the excitation energy from glass matrix to the crystalline component and at the same time about the absence of competition between luminescence processes in both components of the investigated samples with the excitation within the UV light. The latter property of the synthesized class of doped glass is a promising characteristic for the use of developed materials in lighting devices, as it allows improvement of the spectral distribution of light emission towards white light.
5 Conclusions
The xLi2O–yV2O5–(100 − x − y)B2O3 undoped glass and 47Li2O–2V2O5–50B2O3–1La0.3Eu0.7VO4-doped glass were synthesized and investigated using XRD, IR and UV–Vis spectroscopy and UV band-to-band excitation of luminescence. The synthesized glass samples are characterized by wide band photoluminescence emission with maximum at 570 nm and intensity increased with increase of Li2O concentration. An assumption is made that the observed wideband photoluminescence emission of the glass matrix can appear as a result of recombination processes with participation of self-localized holes in borate networks and electrons trapped on the broken vanadate groups. Crystalline component in the doped glass samples doesn’t effects on luminescence properties of the glass matrix. Intensity of narrowband photoluminescence emission of the crystalline component is up to 10 times higher than the intensity of the glass matrix wide band emission. The synthesized type of the doped glass has characteristics of spectral distribution of light emission promising for the use of developed materials in lighting devices.
Data availability
The datasets generated during and analysed during the current study are not publicly available due to internal rules of data processing of some participating bodies but are available from the corresponding author on reasonable request.
References
A.E. Souza, S.G. Antonio, S.J.L. Ribeiro, D.F. Franco, G. Galeani, T. Cardinal, M. Dussauze, M. Nalin, J. Alloys Compd. 864, 158804 (2021)
L.-Y. Chen, W.-C. Cheng, C.-C. Tsai, J.-K. Chang, Y.-C. Huang, J.-C. Huang, W.-H. Cheng, Opt. Express 22, A671–A678 (2014)
G. Gorni, J.J. Velázquez, J. Mosa, R. Balda, J. Fernández, A. Durán, Y. Castro, Materials 11(2), 212 (2018)
C.A.T. Laia, A. Ruivo, in Fluorescence in Industry. Springer Series on Fluorescence, B. Pedras ed., vol 18. Springer, Cham. https://doi.org/10.1007/4243_2019_12
E. Erol, N. Vahedigharehchopogh, O. Kıbrıslı, M. Ersundu, A.E. Ersundu, J. Phys. 33, 483001 (2021)
J. Ren, X. Lu, C. Lin, R.K. Jain, Opt. Express 28, 21522–21548 (2020)
D. Chen, W. Xiang, X. Liang, J. Zhong, H. Yu, M. Ding, H. Lu, Z. Ji, J. Eur. Ceram. Soc. 35, 859–869 (2015)
M. Bengisu, J. Mater. Sci. 51, 2199–2242 (2016)
O. Majérus, H. Trégouët, D. Caurant, D. Pytalev, J. Non-Cryst Solids 425, 91–102 (2015)
K. Milewska, M. Maciejewski, A. Synak, M. Łapiński, A. Mielewczyk-Gryń, W. Sadowski, B. Kościelska, Materials 14(16), 4490 (2021)
A.C. Wright, Phys. Chem. Glass. 51, 1–39 (2010)
N.M. Bobkova, Glass Ceram. 72, 360–365 (2016)
H. Rowson, Inorganic Glass-Forming Systems (New York Academic Press, London, 1967), p.317
B. Saddeek, M.S. Gaafar, Bull. Mater. Sci. 37, 661–667 (2014)
T. Nishida, S. Kubuki, K. Matsuda, Y. Otsuka, Croat. Chem. Acta 88(4), 427–435 (2015)
T. Voitenko, O. Chukova, S.A. Nedilko, S.G. Nedilko, M. Androulidaki, A. Manousaki, E. Stratakis, 2020 IEEE 40th International Conference on Electronics and Nanotechnology (EL NANO), 168–172 (2020)
D. Ehrt, IOP Conf. Ser. 2, 012001 (2009)
O. Chukova, S.G. Nedilko, V. Scherbatskyi, Acta Phys. Pol., A 141, 345–350 (2022)
A.I. Ingle, H.D. Shashikala, M.K. Narayanan, M.T. Dubeto, S. Gupta, Results Eng. 17, 100784 (2023)
A.M. Abdelghany, A.H. Hammad, Spectrochim. Acta A 137, 39–44 (2015)
M.K. Narayanan, H.D. Shashikala, Procedia Mater. Sci. 5, 303–310 (2014)
R.S. Omar, M.A. Saeed, H. Wagiran, H.K. Obayes, PERINTIS eJournal 6(2), 91–100 (2016)
A. Yadav, P. Narwal, M.S. Dahiya, T. Dahiya, A. Agarwal, S. Khasa, AIP Conf. Proc. 1953, 090013 (2018)
A.A. Soliman, Armen. J. Phys. 1, 188–197 (2008)
J. Coelho, C. Freire, N.S. Hussain, Spectrochim. Acta A 86, 392–382 (2012)
S. Kojima, Solids 1, 16–30 (2020)
E.I. Kamitsos, G.D. Chryssikos, M.A. Karakassides, J. Non Cryst. Solids 123, 283–285 (1990)
E.I. Kamitsos, A.P. Patsis, M.A. Karakassides, G.D. Chryssikos, J. Non Cryst. Solids 126, 52–67 (1990)
P.J. Bray, J. Non Cryst. Solids 95–96, 45–60 (1987)
L. Balachander, G. Ramadevudu, Md Shareefuddin, R. Sayanna, Y.C. Venudhar, Sci. Asia 39, 278–283 (2013)
C. Gautam, A. Kumar, A. Yadav, A. Kumar, Singh, ISRN Ceramics, 428497–17 (2012)
Y. Wang, F. Rosowski, R. Schlögl, A. Trunschke, J. Phys. Chem. C 126, 7, 3443–3456 (2022)
Y. Fujikata, F. Ota, K. Hatada, P. Krüger, Phys. Rev. B 101, 125124 (2020)
O. Chukova, S.A. Nedilko, S.G. Nedilko, T. Voitenko, A. Slepets, M. Androulidaki, A. Papadopoulos, E. Stratakis, W. Paszkowicz, J. Lumin. 242, 118587 (2022)
O. Chukova, S.A. Nedilko, S.G. Nedilko, A. Slepets, T. Voitenko, M. Androulidaki, A. Papadopoulos, E. Stratakis, in Springer Proceedings in Physics, vol. 221 (2019), pp. 211–241
G. Malashkevich, Yu Bokshits, O. Chukova, V. Koukhar, S. Nedilko, G. Shevchenko, J. Phys. Chem. C 120, 15369–15377 (2016)
F.H. ElBatal, S.Y. Marzouk, F.M. Ezz-ElDin, J. Mol. Struct. 986, 22–29 (2011)
O.L.G. Alderman, C.J. Benmore, A. Lin, A. Tamalonis, J.K.R. Weber, J. Am. Ceram. Soc. 101, 3357–3371 (2018)
R. Kerner, D.M. dos Santos-Loff, A.C. Rosa, eprint arXiv:2209.01573 (2022)
T. Visser, J.M. Stevels, J. Non Cryst. Solids 7, 376–394 (1972)
R. Kerner, J. Non Cryst. Solids 135, 155–170 (1991)
W.M. Pontuschka, L.S. Kanashiro, L.C. Courrol, Glass Phys. Chem 27, 37–47 (2001)
Acknowledgements
This work was supported by Ministry of Education and Science of Ukraine. Oksana Chukova thanks Deutsches Elektronen-Synchrotron DESY, Hamburg, Germany for possibility of research stay and work in Photon Science group. Serhii Nedilko thanks Polish Academy of Sciences for possibility of research stay and spectroscopy measurements in Institute of Physics PAN, Warsaw.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
SN and OC contributed to the study conception. All authors were engaged to design of the manuscript. The samples were prepared by OC and SN, XRD data collection and analysis were performed by RM and WP, IR data collection and analysis were performed by TV, absorption spectroscopy and analysis were performed by OC, VS and YZ, luminescence spectroscopy and analysis were performed by OC, SN, YZ and AS. The first draft of the manuscript was written by OC and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Research involving human participants or animals
This paper contains no research involving human participants and/or animals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chukova, O., Nedilko, S.G., Voitenko, T. et al. Luminescence mechanisms in the 2V2O5–xLi2O–(98 − x)B2O3 glass matrices developed for creation of glass–ceramic materials. J Mater Sci: Mater Electron 34, 651 (2023). https://doi.org/10.1007/s10854-023-10026-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-10026-4