Abstract
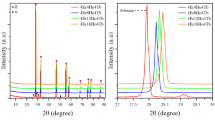
Bi2O3–based solid solutions containing rare earth oxides (Er2O3, Eu2O3, Gd2O3, and Ho2O3) have been produced through solid state reactions under atmospheric conditions. To study the impact of dopant concentration on phase structure and conductivity, the total dopant percentage is increased from 20 to 80%. According to the XRD patterns, only samples A2 (10%Er: 10%Eu: 10%Gd: 05%Ho) and B1 (05%Er: 05%Eu: 05%Gd: 10%Ho) are stabilized by the cubic δ–phase, indicating a homogeneous phase. During heating, the DTA curve of sample A1 (05%Er: 05%Eu: 05%Gd: 05%Ho) displays an endothermic peak at around 729 °C, indicating the phase transition from α–phase to δ–phase. The temperature dependent conductivity graph of the same sample confirm the phase transition due to a sudden increase in conductivity at that temperature value. At 750 °C, sample A2 has the highest conductivity and the lowest activation energy, with values of 0.0144 S.cm–1 and 0.48 eV, respectively. FE–SEM images indicate that the grain sizes are not uniform and decreases as the dopant concentration increases.





Similar content being viewed by others
Data availability
We declare that data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
N. Mahato, A. Banerjee, A. Gupta et al., Progress in material selection for solid oxide fuel cell technology: a review. Prog. Mater. Sci. 72, 141–337 (2015). https://doi.org/10.1016/j.pmatsci.2015.01.001
J. Zhang, C. Lenser, N.H. Menzler et al., Comparison of solid oxide fuel cell (SOFC) electrolyte materials for operation at 500 °C. Solid State Ion. 344, 115–138 (2020). https://doi.org/10.1016/j.ssi.2019.115138
E.D. Wachsman, K.T. Lee, Lowering the temperature of solid oxide fuel cells. Science 334, 935–939 (2011). https://doi.org/10.1126/science.1204090
X. Wang, W. Zhou, J.B. DeLisio et al., Doped δ–bismuth oxides to investigate oxygen ion transport as a metric for condensed phase thermite ignition. Phys. Chem. Chem. Phys. 19, 12749–12758 (2017). https://doi.org/10.1039/C6CP08532F
Z. Zakaria, Z.A. Mat, S. Hasmady et al., A review of solid oxide fuel cell component fabrication methods toward lowering temperature. Int. J. Energy Res. 44, 594–611 (2019). https://doi.org/10.1002/er.4907
A. Dapčević, D. Poleti, J. Rogan et al., A new electrolyte based on Tm3+–doped δ–Bi2O3–type phase with enhanced conductivity. Solid State Ion. 280, 18–23 (2015). https://doi.org/10.1016/j.ssi.2015.08.004
S. Sanna, V. Esposito, J.W. Andreasen et al., Enhancement of the chemical stability in confined δ–Bi2O3. Nat. Mater. 14, 500–504 (2015). https://doi.org/10.1038/nmat4266
H.T. Ozlu, S. Cakar, E. Ersoy et al., The bulk electrical conductivity properties of d–Bi2O3 solid electrolyte system doped with Yb2O3. J. Therm. Anal. Calorim. 122, 525–536 (2015). https://doi.org/10.1007/s10973-015-4785-8
S. Bandyopadhyay, A. Dutta, Thermal, optical and dielectric properties of phase stabilized δ–Dy–Bi2O3 ionic conductors. J. Phys. Chem. Sol. 102, 12–20 (2017). https://doi.org/10.1016/j.jpcs.2016.11.001
Y.S. Ayhan, A. Buyukaksoy, Impact of fabrication temperature on the stability of yttria doped bismuth oxide ceramics. Solid State Ion. 338, 66–73 (2019). https://doi.org/10.1016/j.ssi.2019.05.013
M. Arı, M. Balcı, Y. Polat, Synthesis and characterization of (Bi2O3)1–x−y−z(Gd2O3)x (Sm2O3)y(Eu2O3)z quaternary solid solutions for solid oxide fuel cell. Chin. J. Phys. 56, 2958–2966 (2018). https://doi.org/10.1016/j.cjph.2018.10.001
P. Temluxamea, N. Laosiripojana, S. Assabumrungrata et al., Phase transformation and electrical properties of bismuth oxide doped scandium cerium and gadolinium stabilized zirconia (0.5Gd0.5Ce10ScSZ) for solid oxide electrolysis cell. Int. J. Hydrog. Energy. 45, 29953–29965 (2020). https://doi.org/10.1016/j.ijhydene.2020.08.085
S. Cobaner, S. Yilmaz, Electrical and structural properties of new type Er and Yb doped bismuth oxide solid electrolytes synthesized by Pechini method. J. Electroceramics. 46, 83–92 (2021). https://doi.org/10.1007/s10832-021-00248-5
M. Balci, A. Cengel, M. Ari, The microstructure and thermo–electrical characterization of the Tb–Gd–Ho co–doped stabilized Bi2O3 based solid electrolyte systems. Chin. J. Phys. 79, 89–97 (2022). https://doi.org/10.1016/j.cjph.2022.08.005
E.D. Wachsman, S. Boyapati, N. Jiang, Effect of dopant polarizability on oxygen sublattice order in phase–stabilized cubic bismuth oxides. Ionics 7, 1–6 (2001). https://doi.org/10.1007/BF02375460
S. Dilpuneet, J.C. Aidhy, B.N. Susan et al., Vacancy-ordered structure of cubic bismuth oxide from simulation and crystallographic analysis. J. Am. Ceram. Soc. 91, 2349–2356 (2008). https://doi.org/10.1111/j.1551-2916.2008.02463.x
S.F. Mansour, S. Wageh, M.F. Alotaibi et al., Impact of bismuth oxide on the structure, optical features and ligand field parameters of borosilicate glasses doped with nickel oxide. Ceram. Int. 47, 21443–21449 (2021). https://doi.org/10.1016/j.ceramint.2021.04.154
E. Ersoy, S. Cakar, E. Yildiz et al., Fabrication and characterization of dysprosium-doped bismuth oxide films for IT–SOFCs via slurry spin coating technique. Int. J. Appl. Ceram. Technol. 12, 152–161 (2015). https://doi.org/10.1111/ijac.12387
D.W. Jung, K.L. Duncan, E.D. Wachsman, Effect of total dopant concentration and dopant ratio on conductivity of (DyO15)x–(WO3)y–(BiO15)1–x−y. Acta Mater 58, 355–363 (2010). https://doi.org/10.1016/j.actamat.2009.08.072
S. Bandyopadhyay, A. Dutta, A structural insight into the electrical properties of Dy–Ho co–doped phase stabilized bismuth oxide based electrolytes. J. Electroanal. Chem. 817, 55–64 (2018). https://doi.org/10.1016/j.jelechem.2018.03.063
S. Yilmaz, B. Kavici, C. Celen et al., Structure and conductivity characterization of new type ionic conductor stabilized bismuth oxide ternary systems. Chin. J. Phys. 56, 362–373 (2018). https://doi.org/10.1016/j.cjph.2017.11.010
M.V. Muthamma, S.G. Bubbly, S.B. Gudennavar et al., Poly (vinyl alcohol)–bismuth oxide composites for X–ray and γ–ray shielding applications. J. Appl. Polym. Sci. 136, 47949 (2019). https://doi.org/10.1002/app.47949
M. Vashista, S. Paul, Correlation between full width at half maximum (FWHM) of XRD peak with residual stress on ground surfaces. Philos. Mag. Lett. 92, 4194–4204 (2012). https://doi.org/10.1080/14786435.2012.704429
D.W. Jung, C.N. Juan, K.L. Duncan et al., Enhanced long–term stability of bismuth oxide–based electrolytes for operation at 500 °C. Ionics 16, 97–103 (2010). https://doi.org/10.1007/s11581-009-0402-9
S. Arasteha, A. Maghsoudipour, M. Alizadeh et al., Effect of Y2O3 and Er2O3 co–dopants on phase stabilization of bismuth oxide. Ceram. Int. 37, 3451–3455 (2011). https://doi.org/10.1016/j.ceramint.2011.04.136
A. Güldeste, M. Aldoori, M. Balci et al., Synthesis and characterization of Dy–Eu–Tm co–doped cubic phase stabilized bismuth oxide based electrolytes in terms of intermediate temperature–solid oxide fuel cells (IT–SOFCs). J. Rare Earths. In press (2022). https://doi.org/10.1016/j.jre.2022.03.013
Y. Huang, T. Zhang, Z. Dou et al., Microwave strengthens decomposition of mixed rare earth concentrate: microwave absorption characteristics. J. Rare Earths (2019). https://doi.org/10.1016/j.jre.2018.08.010
Y. Huang, T. Zhang, Z. Dou et al., A review: a new insight for electronic polarizability and chemical bond strength in Bi2O3–based glasses. J. Non-Cryst. Solids. 550, 120365 (2020). https://doi.org/10.1016/j.jnoncrysol.2020.120365
P.S. Cardenas, M.T. Ayala, J. Muñoz et al., High ionic conductivity dysprosium and tantalum Co–doped bismuth oxide electrolyte for low–temperature SOFCs. Ionics 26, 4579–4586 (2020). https://doi.org/10.1007/s11581-020-03572-y
Y. Hua, D. Li, F. Sun et al., Temperature–induced phase changes in bismuth oxides and efficient photodegradation of phenol and p–chlorophenol. J. Hazard. Mater. 301, 362–370 (2016). https://doi.org/10.1016/j.jhazmat.2015.09.008
M. Krynskia, W. Wrobela, C.E. Mohnb et al., Trapping of oxide ions in δ–Bi3YO6. Solid State Ion. 264, 49–53 (2014). https://doi.org/10.1016/j.ssi.2014.06.019
X. Li, Y. Sun, T. Xiong et al., Activation of amorphous bismuth oxide via plasmonic Bi metal for efficient visible–light photocatalysis. J. Catal. 352, 102–112 (2017). https://doi.org/10.1016/j.jcat.2017.04.025
Z. Wang, H. Chen, W. Nian et al., Bismuth oxide modified europium and niobium co–doped titanium dioxide ceramics: colossal permittivity and low dielectric loss design. J. Alloys Compd. 777, 317–324 (2019). https://doi.org/10.1016/j.jallcom.2018.10.366
T. Subburaj, K. Prasanna, K.J. Kim et al., Structural and electrochemical evaluation of bismuth doped lithium titanium oxides for lithium ion batteries. J. Power Sour. 280, 23–29 (2015). https://doi.org/10.1016/j.jpowsour.2015.01.069
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
MB: Data Processing, Software, Methodology, Writing, Analysis, Material Preparation, Experimental Study. MA: Reviewing and Editing.
Corresponding author
Ethics declarations
Conflict of interest
We should declare that the article is original, the article has been written by the stated authors, who are all aware of its content and approve its submission, the article has not been published previously, the article is not under consideration for publication elsewhere and no competing of interest exists in the manuscript. We should also declare that if the manuscript accepted, the article will not be published elsewhere in the same form, in any language, without the written content of the publisher.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Balci, M., Ari, M. A study of the microstructure and thermo–electrical properties of Bi2O3 ceramics co–doped with rare earth oxides. J Mater Sci: Mater Electron 34, 534 (2023). https://doi.org/10.1007/s10854-023-09944-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-09944-0