Abstract
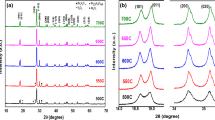
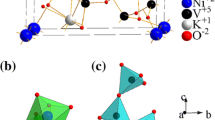
Metal vanadates (MVO) have attained a lot of consideration in recent years because of their unique structural and photoluminescence features. These metal vanadates adopt structures with different phases depending on the magnitude of the bivalent present. Here, the hydrothermal approach was used to synthesize three different phases of MVO (M=Bi, Fe, and Zn). After synthesis temperatures of 200 °C for 24 h, powders of BiVO4 (monoclinic), FeVO4 (anorthic), and ZnV2O4 (cubic) were produced. Scherrer’s evaluation confirmed that these crystallites were nanosized. According to BET data, ZnV2O4 has a sufficient surface area. The bandgap transition of metal vanadates is of the order of FeVO4 < BiVO4 < ZnV2O4 based on UV–Vis spectra. These MVO have interesting optical properties, and photoluminescence (PL) analysis revealed blue-green emission peaks in PL spectra and other obtained results exposed that crystallinity, shape, and size of the metal vanadates have a significant impact on the photocatalytic activity. The photocatalytic degradation of crystal violet (C25H30ClN3l) dye over these hydrothermally synthesized MVO was investigated. In this research, ZnV2O4 spinel oxide offered the best photodegradation efficiency of crystal violet at 99.92% within 60 min under visible light irradiation. The photocatalytic mechanism of this MVO was postulated and carefully reviewed.










Similar content being viewed by others
Data availability
All the data generated or analyzed during this study have been included in this published article.
References
P.L. Ngo et al., Pathway, classification and removal efficiency of microplastics in wastewater treatment plants. Environ. Pollut. 255, 113326 (2019)
S. Ahmed et al., Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J. Hazard. Mater. 416, 125912 (2021)
A.H. Shah, M.A. Rather, Pharmaceutical residues: new emerging contaminants and their mitigation by nano-photocatalysis. Adv. Nano. Res. 10(4), 397–414 (2021)
P. Singh et al., Review on various strategies for enhancing photocatalytic activity of graphene based nanocomposites for water purification. Arab. J. Chem. 13(1), 3498–3520 (2020)
G. Chandrappa et al., Hydrothermal synthesis of vanadium oxide nanotubes from V2O5 gels. Catal. Today 78(1–4), 85–89 (2003)
W.G. Menezes et al., V2O5 nanoparticles obtained from a synthetic bariandite-like vanadium oxide: synthesis, characterization and electrochemical behavior in an ionic liquid. J. Coll. Interface Sci. 337(2), 586–593 (2009)
X.-C. Dai et al., Electrochemically anodized one-dimensional semiconductors: a fruitful platform for solar energy conversion. J. Phys.: Energy 17, 022002 (2019)
K. Kannan et al., Nanostructured metal oxides and its hybrids for photocatalytic and biomedical applications. Adv. Coll. Interface. Sci. 281, 102178 (2020)
M.S.S. Danish et al., Photocatalytic applications of metal oxides for sustainable environmental remediation. Metals 11(1), 80 (2021)
R. Andreozzi et al., Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 53(1), 51–59 (1999)
Y.-J. Zhu, F. Chen, Microwave-assisted preparation of inorganic nanostructures in liquid phase. Chem. Rev. 114(12), 6462–6555 (2014)
A. Heydari et al., Nanorods of FeVO4: An efficient heterogeneous catalyst for chemoselective oxidation of benzylic alcohols. Inorg. Nano-Metal Chem. 47(2), 248–255 (2017)
G. Fey, K. Wang, S. Yang, New inverse spinel cathode materials for rechargeable lithium batteries. J. Power Sources 68(1), 159–165 (1997)
M. Mosleh, Nanocrystalline iron vanadate: facile morphology-controlled preparation, characterization and investigation of optical and photocatalytic properties. J. Mater. Sci.: Mater. Electron. 28(8), 5866–5871 (2017)
R. Monsef, M. Salavati-Niasari, Hydrothermal architecture of Cu5V2O10 nanostructures as new electro-sensing catalysts for voltammetric quantification of mefenamic acid in pharmaceuticals and biological samples. Biosens. Bioelectron. 178, 113017 (2021)
X. Zhang et al., Co2V2O7 particles with intrinsic multienzyme mimetic activities as an effective bioplatform for ultrasensitive fluorometric and colorimetric biosensing. ACS Appl. Bio Mater. 3(3), 1469–1480 (2020)
G. Kesavan, V. Vinothkumar, S.-M. Chen, Sonochemical synthesis of copper vanadate nanoparticles for the highly selective voltammetric detection of antibiotic drug ornidazole. J. Alloy. Compd. 867, 159019 (2021)
M. Tayyab et al., Simultaneous hydrogen production with the selective oxidation of benzyl alcohol to benzaldehyde by a noble-metal-free photocatalyst VC/CdS nanowires. Chin. J. Catal. 43(4), 1165–1175 (2022)
G. Pekar et al., Structural, optical and magnetic properties of stencil-free printed ZnO layers doped with Fe2+ and Fe3+ ions. Mater. Chem. Phys. 276, 125329 (2022)
Z. Feng et al., Self-activated and bismuth-related photoluminescence in rare-earth vanadate, niobate, and tantalate series—a first-principles study. Inorg. Chem. 60(21), 16614–16625 (2021)
K. Anwar, F.K. Naqvi, S. Beg, Synthesis of tetragonally stabilized lanthanum doped bismuth vanadium oxide nanoparticles and its enhanced visible light induced photocatalytic performance. Phase Transitions 95(1), 64–79 (2022)
N.G. Chernorukov et al., A family of uranyl oxide hydrate phases with bivalent cations [M II (H 2 O) 4][(UO 2) 3 O 3 (OH) 2]· H 2 O (M II–Mn Co, Ni, Zn): synthesis, characterization and chemical stability in aqueous solutions. New J. Chem. 45(22), 9922–9935 (2021)
M.M. Sajid et al., Hydrothermal fabrication of monoclinic bismuth vanadate (m-BiVO4) nanoparticles for photocatalytic degradation of toxic organic dyes. Mater. Sci. Eng., B 242, 83–89 (2019)
M.M. Sajid et al., Facile synthesis of Se/BiVO4 heterojunction composite and evaluation of synergetic reaction mechanism for efficient photocatalytic staining of organic dye pollutants in wastewater under visible light. J. Mater. Sci.: Mater. Electron. 31(22), 19599–19612 (2020)
S. Tokunaga, H. Kato, A. Kudo, Selective preparation of monoclinic and tetragonal BiVO4 with scheelite structure and their photocatalytic properties. Chem. Mater. 13(12), 4624–4628 (2001)
R. Yang et al., One-step preparation (3D/2D/2D) BiVO4/FeVO4@ rGO heterojunction composite photocatalyst for the removal of tetracycline and hexavalent chromium ions in water. Chem. Eng. J. 390, 124522 (2020)
H.-Q. Jiang et al., Fabrication and photoactivities of spherical-shaped BiVO4 photocatalysts through solution combustion synthesis method. J. Eur. Ceram. Soc. 28(15), 2955–2962 (2008)
C. Hong et al., Comprehensive study of the growth mechanism and photoelectrochemical activity of a BiVO4/Bi2S3 nanowire composite. ACS Appl. Mater. Interfaces. 12(35), 39713–39719 (2020)
M.M. Sajid et al., Photocatalytic performance of ferric vanadate (FeVO4) nanoparticles synthesized by hydrothermal method. Mater. Sci. Semicond. Process. 129, 105785 (2021)
M.M. Sajid et al., Morphological effects on the photocatalytic performance of FeVO4 nanocomposite. Nano-Structures & Nano-Objects 22, 100431 (2020)
L.M.Z. De Juan-Corpuz et al., Porous ZnV2O4 nanowire for stable and high-rate lithium-ion battery anodes. ACS Applied Nano Materials 2(7), 4247–4256 (2019)
J. Duan et al., Construction of columnar cactus-like 2D/1D CdxCu1-xS@ CuO shell-core structure photocatalyst for the reduction of CO2 to methanol. Opt. Mater. 115, 111016 (2021)
F. Duan et al., Template-free synthesis of ZnV2O4 hollow spheres and their application for organic dye removal. Appl. Surf. Sci. 258(1), 189–195 (2011)
S. Kawaguchi, Orbital order and phase transitions in vanadium spinels. Phys. Rev. B 87, 054432 (2013)
Krimmel, A., Electronically highly correlated ternary transition metal oxides. 2005: diplom. de.
C. Carrero et al., Critical literature review of the kinetics for the oxidative dehydrogenation of propane over well-defined supported vanadium oxide catalysts. ACS Catal. 4(10), 3357–3380 (2014)
Y. He et al., Influence of catalyst synthesis method on selective catalytic reduction (SCR) of NO by NH3 with V2O5-WO3/TiO2 catalysts. Appl. Catal. B 193, 141–150 (2016)
B.M. Weckhuysen, D.E. Keller, Chemistry, spectroscopy and the role of supported vanadium oxides in heterogeneous catalysis. Catal. Today 78(1–4), 25–46 (2003)
M.M. Sajid et al., Visible light assisted photocatalytic degradation of crystal violet dye and electrochemical detection of ascorbic acid using a BiVO 4/FeVO 4 heterojunction composite. RSC Adv. 8(42), 23489–23498 (2018)
M.M. Sajid et al., Study of the interfacial charge transfer in bismuth vanadate/reduce graphene oxide (BiVO4/rGO) composite and evaluation of its photocatalytic activity. Res. Chem. Intermed. 46(2), 1201–1215 (2020)
F.K. Butt et al., Synthesis, evolution and hydrogen storage properties of ZnV2O4 glomerulus nano/microspheres: a prospective material for energy storage. Int. J. Hydrogen Energy 39(15), 7842–7851 (2014)
X. Zhu et al., Nanophase ZnV2O4 as stable and high capacity Li insertion electrode for Li-ion battery. Curr. Appl. Phys. 15(4), 435–440 (2015)
M. Umar et al., Rationally designed La and Se co-doped bismuth ferrites with controlled bandgap for visible light photocatalysis. RSC Adv. 9(30), 17148–17156 (2019)
M.M. Sajid et al., Construction of 1T-MoS 2 quantum dots-interspersed (Bi 1–x Fe x) VO 4 heterostructures for electron transport and photocatalytic properties. RSC Adv. 11(22), 13105–13118 (2021)
S. Irfan et al., The gadolinium (Gd3+) and Tin (Sn4+) Co-doped BiFeO3 nanoparticles as new solar light active photocatalyst. Sci. Rep. 7(1), 1–12 (2017)
N.A. Shad et al., Photocatalytic investigation of cadmium oxide nanosheets prepared by hydrothermal method. Arabian J. Sci. Eng. (2019). https://doi.org/10.1007/s13369-019-03897-5
N.A. Shad et al., Photocatalytic degradation performance of cadmium tungstate (CdWO4) nanosheets-assembly and their hydrogen storage features. Ceram. Int. (2019). https://doi.org/10.1016/j.ijhydene.2021.05.211
Q.A. Alsulami et al., Preparation of highly efficient sunlight driven photodegradation of some organic pollutants and H2 evolution over rGO/FeVO4 nanocomposites. Int. J. Hydrogen Energy 46(54), 27349–27363 (2021)
Y.M. Hunge et al., Visible light-assisted photocatalysis using spherical-shaped bivo4 photocatalyst. Catalysts 11(4), 460 (2021)
M. Tahir, Hierarchical 3D VO2/ZnV2O4 microspheres as an excellent visible light photocatalyst for CO2 reduction to solar fuels. Appl. Surf. Sci. 467, 1170–1180 (2019)
F.F. Abdi et al., Spray-deposited Co-Pi Catalyzed BiVO 4: a low-cost route towards highly efficient photoanodes. MRS Online Proc. Lib. Archive (2012). https://doi.org/10.1557/opl.2012.811
Y. Liu et al., Single-atom Pt loaded zinc vacancies ZnO–ZnS induced type-V electron transport for efficiency photocatalytic h2 evolution. Solar Rrl 5(11), 2100536 (2021)
J. Deng et al., FeVO4 as a highly active heterogeneous Fenton-like catalyst towards the degradation of orange II. Appl. Catal. B 84(3–4), 468–473 (2008)
S.A. Bakar, C. Ribeiro, An insight toward the photocatalytic activity of S doped 1-D TiO2 nanorods prepared via novel route: as promising platform for environmental leap. J. Mol. Catal. A: Chem. 412, 78–92 (2016)
M.M. Sajid et al., Platinum doped bismuth vanadate (Pt/BiVO4) for enhanced photocatalytic pollutant degradation using visible light irradiation. J. Mater. Sci.: Mater. Electron. (2022). https://doi.org/10.1007/s10854-022-08431-2
W. Tress, Perovskite solar cells on the way to their radiative efficiency limit–insights into a success story of high open-circuit voltage and low recombination. Adv. Energy Mater. 7(14), 1602358 (2017)
S. Irfan et al., Band-gap engineering and enhanced photocatalytic activity of Sm and Mn doped BiFeO3 nanoparticles. J. Am. Ceram. Soc. 100(1), 31–40 (2017)
M. Kiani, S. Rizwan, S. Irfan, Facile synthesis of a BiFeO3/nitrogen-doped graphene nanocomposite system with enhanced photocatalytic activity. J. Phys. Chem. Solids 121, 8–16 (2018)
M.M. Sajid et al., Preparation and characterization of Vanadium pentoxide (V2O5) for photocatalytic degradation of monoazo and diazo dyes. Surfaces Interfaces (2020). https://doi.org/10.1016/j.surfin.2020.100502
M.M. Sajid et al., Synthesis of novel visible light assisted Pt doped zinc vanadate (Pt/Zn4V2O9) for enhanced photocatalytic properties. Chem. Phys. 539, 110980 (2020)
M.M. Sajid et al., Generation of strong oxidizing radicals from plate-like morphology of BiVO4 for the fast degradation of crystal violet dye under visible light. Appl. Phys. A 126(4), 314 (2020)
S. Irfan et al., Enhanced photocatalytic activity of La 3+ and Se 4+ co-doped bismuth ferrite nanostructures. J. Mater. Chem. A 5(22), 11143–11151 (2017)
M. Tayyab et al., One-pot in-situ hydrothermal synthesis of ternary In2S3/Nb2O5/Nb2C Schottky/S-scheme integrated heterojunction for efficient photocatalytic hydrogen production. J. Coll. Interface Sci. 628, 500–512 (2022)
M.M. Sajid et al., Facile synthesis of Zinc vanadate Zn3 (VO4) 2 for highly efficient visible light assisted photocatalytic activity. J. Alloy. Compd. 775, 281–289 (2019)
B. Zhang et al., Quantitative investigation into the enhancing utilization efficiency of H2O2 catalyzed by FeOCl under visible light. J. Photochem. Photobiol., A 386, 112072 (2020)
M. Mittal, M. Sharma, O. Pandey, Fast and quick degradation properties of doped and capped ZnO nanoparticles under UV–Visible light radiations. Sol. Energy 125, 51–64 (2016)
M.M. Sajid et al., Generation of strong oxidizing radicals from plate-like morphology of BiVO4 for the fast degradation of crystal violet dye under visible light. Appl. Phys. A 126(4), 1–12 (2020)
M.Y.A. Khan et al., Visible light photocatalytic degradation of crystal violet dye and electrochemical detection of ascorbic acid & glucose using BaWO4 nanorods. Mater. Res. Bull. 104, 38–43 (2018)
M.K. Seery et al., Silver doped titanium dioxide nanomaterials for enhanced visible light photocatalysis. J. Photochem. Photobiol., A 189(2–3), 258–263 (2007)
S. Adhikari et al., Visible light assisted improved photocatalytic activity of combustion synthesized spongy-ZnO towards dye degradation and bacterial inactivation. RSC Adv. 6(83), 80086–80098 (2016)
S. Senthilkumaar, K. Porkodi, Heterogeneous photocatalytic decomposition of crystal violet in UV-illuminated sol–gel derived nanocrystalline TiO2 suspensions. J. Coll. Interface Sci. 288(1), 184–189 (2005)
Acknowledgements
Dr. Muhammad Munir Sajid thanks Dr. Zhengjun Zhang of Tsinghua University in Beijing, China, for his assistance with characterization procedures. Dr. Muhammad Munir Sajid also acknowledges the financial support by the State Scholarship Fund of China Scholarship Council (Grant No. 201808410144), the National Natural Science Foundation of China (Grant No. 51202107), and the Foundation of Henan Educational Committee (Grant No. 20A480003).
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
MMS contributed to methodology, material experiments, writing original draft preparation, material characterization, photocatalysis data analysis, and investigation; HA contributed to reviewing, editing, conceptualization, methodology, and sources. HZ contributed to material characterization, reviewing, and editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicting interests that are relevant to the content of this work to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sajid, M.M., Assaedi, H. & Zhai, H. Transition metal vanadates (MVO; M=Bi, Fe, Zn) synthesized by a hydrothermal method for efficient photocatalysis. J Mater Sci: Mater Electron 34, 539 (2023). https://doi.org/10.1007/s10854-023-09923-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-023-09923-5