Abstract
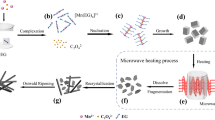
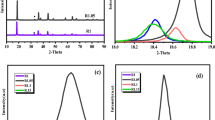
Manganese oxalate, a cheap anode material for lithium-ion batteries, suffers from a low actual capacity due to its low conductivity. To improve its electrochemical performance, a strategy based on adjusting the reaction temperature was proposed to controllably synthesize manganese oxalates with various morphologies. When the reaction temperature is lower than the boiling point of ethylene glycol, the growth process of manganese oxalate is dominated by the dissolution. The manganese oxalate crystal maintains its initial cubic shape, but its size decreases. When the reaction temperature is higher than the boiling point of ethylene glycol, the initial cubes gradually change into rods through dissolution–recrystallization-oriented growth processes under the high-temperature/pressure environment. The specific surface area and the pore volume of manganese oxalate increase first and then decrease with the increase in reaction temperature. MnC2O4 prepared at 220 °C is a mesoporous rod-shaped particle with the highest specific surface area and pore volume. This sample delivers a capacity of 972 and 949 mAh/g after 500 cycles at 2 and 5 A/g, respectively, exhibiting high specific capacity and good cyclic stability. These results show that the reaction temperature can control the morphology of manganese oxalate by adjusting the crystal growth process, thereby changing its electrochemical properties. Therefore, the results provided further confirm the effectiveness of the proposed strategy.








Similar content being viewed by others

Data availability
All data generated or analyzed during this study are included in this published article.
References
A. Manthiram, Nat. Commun. 11, 1550 (2020). https://doi.org/10.1038/s41467-020-15355-0
A. Tomaszewska, Z. Chu, X. Feng, S. O’Kane, X. Liu, J. Chen, C. Ji, E. Endler, R. Li, L. Liu, Y. Li, S. Zheng, S. Vetterlein, M. Gao, J. Du, M. Parkes, M. Ouyang, M. Marinescu, G. Offer, B. Wu, eTransportation 1, 100011 (2019). https://doi.org/10.1016/j.etran.2019.100011
X. Han, L. Lu, Y. Zheng, X. Feng, Z. Li, J. Li, M. Ouyang, eTransportation 1, 100005 (2019). https://doi.org/10.1016/j.etran.2019.100005
P.G. Bruce, B. Scrosati, J. Tarascon, Angew. Chem. Int. Ed. 47, 2930–2946 (2008). https://doi.org/10.1002/anie.200702505
A. Manthiram, ACS Cent. Sci. 3, 1063–1069 (2017). https://doi.org/10.1021/acscentsci.7b00288
T.T. Wei, P. Peng, Y.R. Ji, Y.R. Zhu, T.F. Yi, Y. Xie, J. Energy Chem. 71, 400–410 (2022). https://doi.org/10.1016/j.jechem.2022.04.017
T.F. Yi, L. Shi, X. Han, F. Wang, Y. Zhu, Y. Xie, Energy Environ. Mater. 4(4), 586–595 (2021). https://doi.org/10.1002/eem2.12140
T.F. Yi, J. Mei, P.P. Peng, S. Luo, Compos. Part B-Eng. 167, 566–572 (2019). https://doi.org/10.1016/j.compositesb.2019.03.032
F. Zhang, C. Yang, X. Gao, S. Chen, Y. Hu, H. Guan, Y. Ma, J. Zhang, H. Zhou, L. Qi, ACS Appl. Mater. Interfaces 2017(9), 9620–9629 (2017). https://doi.org/10.1021/acsami.6b15880
F. Zhang, C. Yang, H. Guan, Y. Hu, C. Jin, C. Jin, H. Zhou, L. Qi, ACS Appl. Energy Mater. 2018(1), 5417–5427 (2018). https://doi.org/10.1021/acsaem.8b01024
H. Chang, Y.R. Wu, X. Han, T.F. Yi, Energy Mater. 1, 100003 (2021). https://doi.org/10.20517/energymater.2021.02
K. Liu, Y. Liu, D. Lin, A. Pei, C. Yi, Sci. Adv. 4, 9820 (2018). https://doi.org/10.1126/sciadv.aas9820
Y.L. Sui, J. Zhou, X.W. Wang, L. Wu, S.K. Zhong, Y.G. Li, Mater. Today 42, 117–136 (2021). https://doi.org/10.1016/j.mattod.2020.09.005
L. Fang, C. Wang, L. Huangfu, N. Bahlawane, H. Tian, Y. Lu, H. Pan, M. Yan, Y. Jiang, Adv. Funct. Mater. 29, 1906680 (2019). https://doi.org/10.1002/adfm.201906680
N. Li, Q. Li, X. Guo, M. Yuan, H. Pang, Chem. Eng. J. 372, 551–571 (2019). https://doi.org/10.1016/j.cej.2019.04.127
M.C. López, J.L. Tirado, C. Pérez Vicente, J. Power Sources 227, 65–71 (2013). https://doi.org/10.1016/j.jpowsour.2012.08.100
K. Zhang, Y. Li, Y. Wang, J. Zhao, X. Chen, Y. Dai, Y. Yao, Chem. Eng. J. 384, 123281 (2020). https://doi.org/10.1016/j.cej.2019.123281
K. Zhang, R. Xu, R. Wei, Y. Li, Y. Wang, Y. Zhang, Y. Dai, Y. Yao, Mater. Chem. Phys. 243, 122676 (2020). https://doi.org/10.1016/j.matchemphys.2020.122676
W.A. Ang, Y.L. Cheah, C.L. Wong, R. Prasanth, H.H. Hng, S. Madhavi, J. Phys. Chem. C 117, 16316–16325 (2013). https://doi.org/10.1021/jp404049f
W.A. Ang, N. Gupta, R. Prasanth, S. Madhavi, ACS Appl. Mater. Interfaces 4, 7011–7019 (2012). https://doi.org/10.1021/am3022653
Y. Zhang, S. Li, H. Kuai, Y. Long, X. Lv, J. Su, Y. Wen, RSC Adv. 11, 23259–23269 (2021). https://doi.org/10.1039/D1RA03669F
K. Zhang, D. Cui, X. Huang, F. Liang, G. Gao, T. Song, L. Zhang, Y. Yao, Y. Lei, Chem. Eng. J. 426, 131446 (2021). https://doi.org/10.1016/j.cej.2021.131446
Y. Zhu, F. Chen, Chem. Rev. 114, 6462–6555 (2014). https://doi.org/10.1021/cr400366s
G. Yang, S. Park, Materials 12, 1177 (2019). https://doi.org/10.3390/ma12071177
Q. Zheng, Y. Liu, H. Guo, X. Hua, S. Shi, M. Zuo, Mater. Res. Bull. 98, 155–159 (2018). https://doi.org/10.1016/j.materresbull.2017.10.017
Z. Qi, Y. Wu, X. Li, Y. Qu, Y. Yang, D. Mei, Ionics 26, 33–42 (2020). https://doi.org/10.1007/s11581-019-03181-4
W. Kang, Q. Shen, J. Power Sources 238, 203–209 (2013). https://doi.org/10.1016/j.jpowsour.2013.03.087
W.A.E. Ang, Y.L. Cheah, C.L. Wong, H.H. Hng, S. Madhavi, J. Alloys Compd. 638, 324–333 (2015). https://doi.org/10.1016/j.jallcom.2015.02.203
F. Feng, W. Kang, F. Yu, H. Zhang, Q. Shen, J. Power Sources 282, 109–117 (2015). https://doi.org/10.1016/j.jpowsour.2015.02.043
H. Oh, C. Jo, C.S. Yoon, H. Yashiro, S. Kim, S. Passerini, Y. Sun, S. Myung, NPG Asia Mater. 8, e270 (2016). https://doi.org/10.1038/am.2016.59
Y. Jia, A. Cheng, W. Ke, J. Liu, S. Wang, Y. Zhao, Q. Yang, J. Zhang, Electrochim. Acta 380, 138217 (2021). https://doi.org/10.1016/j.electacta.2021.138217
S. Dąbrowska, T. Chudoba, J. Wojnarowicz, W. Łojkowski, Crystals 8, 379 (2018). https://doi.org/10.3390/cryst8100379
S. Cao, W. Zeng, H. Long, H. Zhang, Mater. Lett. 159, 385–388 (2015). https://doi.org/10.1016/j.matlet.2015.07.045
J. Xu, L. He, H. Liu, T. Han, Y. Wang, C. Zhang, Y. Zhang, Electrochim. Acta 170, 85–91 (2015). https://doi.org/10.1016/j.electacta.2015.04.114
W.Y. Wu, T.H. Tang, Y. Li, S. Xu, Dalton Trans. 50, 485–489 (2021). https://doi.org/10.1039/D0DT03765F
T. Pu, J. Li, Y. Jiang, B. Huang, W. Wang, C. Zhao, L. Xie, L. Chen, Dalton Trans. 47, 9241–9249 (2018). https://doi.org/10.1039/C8DT01920G
M. Muttakin, S. Mitra, K. Thu, K. Ito, B.B. Saha, Int. J. Heat Mass Transf. 122, 795–805 (2018). https://doi.org/10.1016/j.ijheatmasstransfer.2018.01.107
K.S. Sing, D.H. Everett, R.A. Haul, Pure Appl. Chem. 57, 603–619 (1985). https://doi.org/10.1351/pac198557040603
Y. Zhang, Z. Lu, M. Guo, Z. Bai, B. Tang, JOM 68, 2952–2957 (2016). https://doi.org/10.1007/s11837-016-2126-4
B. León, C.P. Vicente, J.L. Tirado, Solid State Ion. 225, 518–521 (2012). https://doi.org/10.1016/j.ssi.2011.12.012
M.J. Aragón, B. León, C. Pérez Vicente, J.L. Tirado, Inorg. Chem. 47, 10366–10371 (2008). https://doi.org/10.1021/ic8008927
M.J. Aragón, B. León, C. Pérez Vicente, J.L. Tirado, A.V. Chadwick, A. Berko, S. Beh, Chem. Mater. 21, 1834–1840 (2009). https://doi.org/10.1021/cm803435p
X. Wu, J. Guo, M.J. McDonald, S. Li, B. Xu, Y. Yang, Electrochim. Acta 163, 93–101 (2015). https://doi.org/10.1016/j.electacta.2015.02.134
P. Simon, Y. Gogotsi, B. Dunn, Science 343, 1210–1211 (2014). https://doi.org/10.1126/science.1249625
X. Pu, D. Zhao, C. Fu, Z. Chen, S. Cao, C. Wang, Y. Cao, Angew. Chem. Int. Ed. 60, 21310–21318 (2021). https://doi.org/10.1002/ange.202104167
Acknowledgements
The authors appreciate the financial support from the National Natural Science Foundation of China (51864005 and 51564002), the Natural Science Foundation of Guangxi, China (2018GXNSFDA281014), and the College Students' Innovative Entrepreneurial Training Plan of Guangxi University (20220100634).
Funding
The funded was provided by the National Natural Science Foundation of China (Grant No: 51864005).
Author information
Authors and Affiliations
Contributions
YXH, DDZ, XYH, XPC, and LXL performed the experiments and characterization of materials. LYX analyzed the data and discussed the results. JS wrote the manuscript. YXW conceived and designed the research and wrote the manuscript. All the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
This manuscript has not been published or presented elsewhere in part or entirety and is not under consideration by another journal. We have read and understood your journal’s policies, and we believe that neither the manuscript nor the study violates any of these. There is no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, YX., Zeng, DD., Huang, XY. et al. Regulating morphology and lithium storage properties of manganese oxalate prepared by optimizing reaction temperature. J Mater Sci: Mater Electron 34, 198 (2023). https://doi.org/10.1007/s10854-022-09645-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-022-09645-0