Abstract
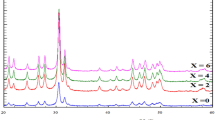
LAMOX are new materials deriving from La2Mo2O9 extensively studied due to their recently discovered high ionic conductivity (better than best stabilized zirconia above 600 °C). So, applications as fuel cell electrolytes can be predicted. Other applications such as electrode materials and oxidation catalysts are also possible to the control of the molybdenum oxidation states. The lanthanum molybdate La2Mo2O9 is taken as a reference in this paper. The transition from a weakly conducting phase (α-La2Mo2O9, monoclinic, ordered) to the highly conducting phase (β-La2Mo2O9, cubic, disordered) occurs at 580 °C. The aim of this work was to generate protonic and anionic conductors. To prepare the composition of the solid solution La2-2x(Mo, S)2O9-3x·xH2O, a powder solid-state reaction pathway was used as a sulfur source. Pure cubic phase (β -form, the space group P213) was obtained when the S6+ contents had reached 50 mol%. The structure and lattice parameters of La2MoSO9 are obtained from Rietveld refinement. The simultaneous DTA and TGA measurements demonstrate thermal stability. The sintered specimens are annealed at 850 °C to test their electrical properties. The temperature dependence of electrical conductivities for La2Mo2O9 and La2MoSO9 obeyed the Arrhenius law below 580 °C, whereas the Vogel-Tammann-Fulcher (VTF) regime could satisfactorily represent the conduction behaviors beyond 580 °C. The infrared and Raman spectra were performed at ambient temperature. All theoretically predicted vibrations have been observed, sulfate ions’ environment influences their internal vibration modes. Investigating the vibrational spectra of these two phases (α and β) can help us to acquire more definitive information on the internal vibrations of XO4 (X=Mo, S).










Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to the reasons of ethics and ownership, but are available from the corresponding author on reasonable request.
References
A.M. Mansour, I.M. El Radaf, T.A. Hameed, G.B. Sakr, Investigation of Ag2HgI4 nanoparticules: thermal phase transition and non-isothermal kinetic study. Bull. Ser. B 81, 134–148 (2019)
A.M. El Nahrawy, A.B.A. Hammad, A.M. Youssef, A.M. Mansour, A.M. Othman, Thermal, dielectric and antimicrobial properties of polystyrene-assisted/ITO: Cu nanocomposites. Appl. Phys. A Mater. Sci. Process. 125, 1–9 (2019). https://doi.org/10.1007/S00339-018-2351-5/TABLES/1
E.M. El-Menyawy, I.T. Zedan, A.M. Mansour, H.H. Nawar, Thermal stability, AC electrical conductivity and dielectric properties of N-(5-{[antipyrinyl-hydrazono]-cyanomethyl}-[1,3,4]thiadiazol-2-yl)-benzamide. J. Alloys Compd. 611, 50–56 (2014). https://doi.org/10.1016/J.JALLCOM.2014.05.120
A.B. Abou Hammad, A.M. Mansour, A.M. El Nahrawy, Ni2+doping effect on potassium barium titanate nanoparticles: enhancement optical and dielectric properties. Phys. Scr. 96, 125821 (2021). https://doi.org/10.1088/1402-4896/AC25A6
A.M. Mansour, I.M. El Radaf, G.M. Mahmoud, Effect of deposition temperature on structural, optical and electrical properties of chemically deposited thermochromic Cu2HgI4 thin films. Int. J. Microstruct. Mater. Prop. 14, 462–477 (2019). https://doi.org/10.1504/IJMMP.2019.102223
A.A.M. Farag, A.M. Mansour, A.H. Ammar, M.A. Rafea, A.M. Farid, Electrical conductivity, dielectric properties and optical absorption of organic based nanocrystalline sodium copper chlorophyllin for photodiode application. J. Alloys Compd. 513, 404–413 (2012). https://doi.org/10.1016/J.JALLCOM.2011.10.058
A.M. El Nahrawy, A.B. Abou Hammad, A.M. Bakr, T.I. Shaheen, A.M. Mansour, Sol–gel synthesis and physical characterization of high impact polystyrene nanocomposites based on Fe2O3 doped with ZnO. Appl. Phys. A Mater. Sci. Process. 126, 1–11 (2020). https://doi.org/10.1007/S00339-020-03822-W/FIGURES/9
T.A. Hameed, F. Mohamed, A.M. Mansour, I.K. Battisha, Synthesis of Sm3+ and Gd3+ ions embedded in nano-structure barium titanate prepared by sol-gel technique: terahertz, dielectric and up-conversion study. ECS J. Solid State Sci. Technol. 9, 123005 (2020). https://doi.org/10.1149/2162-8777/ABC96B
A.M. El Nahrawy, A.I. Ali, A.M. Mansour, A.B. Abou Hammad, B.A. Hemdan, S. Kamel, Talented Bi0.5Na0.25K0.25TiO3/oxidized cellulose films for optoelectronic and bioburden of pathogenic microbes. Carbohydr. Polym. 291, 119656 (2022). https://doi.org/10.1016/J.CARBPOL.2022.119656
R.S. Ibrahim, A.A. Azab, A.M. Mansour, Synthesis and structural, optical, and magnetic properties of Mn-doped CdS quantum dots prepared by chemical precipitation method. J. Mater. Sci. Mater. Electron. 32, 19980–19990 (2021). https://doi.org/10.1007/S10854-021-06522-0/FIGURES/11
A.M. Mansour, F.M.A. El-Taweel, R.A.N. Abu El-Enein, E.M. El-Menyawy, Structural, Optical, Electrical and Photoelectrical Properties of 2-Amino-4-(5-bromothiophen-2-yl)-56-dihydro-6-methyl-5-oxo-4H-pyrano[3,2-c] quinoline-3-carbonitrile Films. J. Electron. Mater. 46(12), 6957–6964 (2017). https://doi.org/10.1007/S11664-017-5739-7
I.T. Zedan, E.M. El-Menyawy, A.M. Mansour, Physical characterizations of 3-(4-Methyl Piperazinylimino Methyl) rifampicin films for photodiode applications. SILICON 113(11), 1693–1699 (2018). https://doi.org/10.1007/S12633-018-9989-7
F. Goutenoire, O. Isnard, R. Retoux, P. Lacorre, Crystal structure of La2Mo2O9, a new fast oxide-ion conductor. Chem. Mater. 12, 2575–2580 (2000). https://doi.org/10.1021/CM991199L/ASSET/IMAGES/LARGE/CM991199LF00008.JPEG
I.R. Evans, J.A.K. Howard, J.S.O. Evans, The crystal structure of α-La2Mo2O9 and the structural origin of the oxide ion migration pathway. Chem. Mater. (2005). https://doi.org/10.1021/CM050049
F. Goutenoire, O. Isnard, E. Suard, O. Bohnke, Structural and transport characteristics of the LAMOX family of fast oxide-ion conductors, based on lanthanum molybdenum oxide La2Mo2O9 basis of a Pubs.Rsc.Org. J. Mater. Chem. 11, 119–124 (2001). https://doi.org/10.1039/B002962I
P. Lacorre, F. Goutenoire, O. Bohnke, R. Retoux, Y. Lallgant, Designing fast oxide-ion conductors based on La2Mo2O9. Nature 404, 856–858 (2000). https://doi.org/10.1038/35009069
S. Georges, F. Goutenoire, O.Bohnke for E., undefined 2004, The LAMOX family of fast oxide-ion conductors: overview and recent results, Academia.Edu. (2022). https://www.academia.edu/download/42653329/The_LAMOX_Family_of_Fast_OxideIon_Conduc_20160213_4521_11e6n6i.pdf. (Accessed 4th Oct, 2022).
X.P. Wang, Q.F. Fang, Z.S. Li, G.G. Zhang, Z.G. Yi, Dielectric relaxation studies of Bi-doping effects on the oxygen-ion diffusion in La2−xBixMo2O9 oxide-ion conductors. Appl. Phys. Lett. 81, 3434 (2002). https://doi.org/10.1063/1.1518151
A. Subramania, T. Saradha, S. Muzhumathi, Synthesis, sinterability and ionic conductivity of nanocrystalline Pr-doped La2Mo2O9 fast oxide-ion conductors. J. Power Sources. 167, 319–324 (2007). https://doi.org/10.1016/J.JPOWSOUR.2007.01.094
S. Georges, F. Goutenoire, F. Altorfer, D. Sheptyakov, F. Fauth, E. Suard, P. Lacorre, Thermal, structural and transport properties of the fast oxide-ion conductors La2−xRxMo2O9 (R=Nd, Gd, Y). Solid State Ionics 161, 231–241 (2003). https://doi.org/10.1016/S0167-2738(03)00279-0
D. Marrero-López, P. Núñez, M. Abril, V. Lavín, U.R. Rodríguez-Mendoza, V.D. Rodríguez, Synthesis, electrical properties, and optical characterization of Eu3+ doped La2Mo2O9 nanocrystalline phosphors. J. Non. Cryst. Solids. 345–346, 377–381 (2004). https://doi.org/10.1016/J.JNONCRYSOL.2004.08.047
C. Tealdi, G. Chiodelli, L. Malavasi, G. Flor, Effect of alkaline-doping on the properties of La2Mo2O9 fast oxygen ion conductor. J. Mater. Chem (2004). https://doi.org/10.1039/b410437d
X.P. Wang, Z.J. Cheng, Q.F. Fang, Influence of potassium doping on the oxygen-ion diffusion and ionic conduction in the La2Mo2O9 oxide-ion conductors. Solid State Ionics 176, 761–765 (2005). https://doi.org/10.1016/J.SSI.2004.10.015
D. Marrero-López, D. Pérez-Coll, J.C. Ruiz-Morales, J. Canales-Vázquez, M.C. Martín-Sedeño, P. Núñez, Synthesis and transport properties in La2−xAxMo2O9−δ (A = Ca2+, Sr2+, Ba2+, K+) series. Electrochim. Acta. 52, 5219–5231 (2007). https://doi.org/10.1016/J.ELECTACTA.2007.02.033
G. Corbel, Y. Laligant, F. Goutenoire, E. Suard, P. Lacorre, Effects of partial substitution of Mo6+ by Cr6+ and W6+ on the crystal structure of the fast oxide-ion conductor structural effects of W6+. Chem. Mater. 17, 4678–4684 (2005). https://doi.org/10.1021/CM0501214/SUPPL_FILE/CM0501214SI20050119_114305.CIF
J.A. Collado, M.A.G. Aranda, A. Cabeza, P. Olivera-Pastor, S. Bruque, Synthesis, Structures, and thermal expansion of the La2W2−xMoxO9 series. J. Solid State Chem. 167, 80–85 (2002). https://doi.org/10.1006/JSSC.2002.9622
N. Mhadhbi, G. Corbel, P. Lacorre, A. Bulou, Partial substitution of Mo6+ by S6+ in the fast oxide ion conductor La2Mo2O9: Synthesis, structure and sulfur depletion. J. Solid State Chem. 190, 246–256 (2012). https://doi.org/10.1016/J.JSSC.2012.02.043
V.I. Voronkova, E.P. Kharitonova, A.E. KrasilNikova, Specific features of phase transitions and the conduction of La2Mo2O9 oxide-ion conducting compound doped with vanadium. Crystallogr. Rep. 55(2), 276–282 (2010). https://doi.org/10.1134/S1063774510020203
S. Basu, P.S. Devi, H.S. Maiti, Nb-Doped La2Mo2O9: a new material with high ionic conductivity. J. Electrochem. Soc. 152, A2143 (2005). https://doi.org/10.1149/1.2047507
Z.S. Khadasheva, N.U. Venskovskii, M.G. Safronenko, A.V. Mosunov, E.D. Politova, S.Y. Stefanovich, Synthesis and properties of La2(Mo1–x Mx)2O9 (M = Nb, Ta) ionic conductors. Inorg. Mater. 38, 1168–1171 (2002). https://doi.org/10.1023/A:1020978902757
R.D. Bayliss, S.N. Cook, S. Kotsantonis, R.J. Chater, J.A. Kilner, Oxygen ion diffusion and surface exchange properties of the α- and δ-phases of Bi2O3. Adv. Energy Mater. 4, 1301575 (2014). https://doi.org/10.1002/AENM.201301575
J.A. Kilner, M. Burriel, Mater. Intermed. Temp. Solid-Oxide Fuel Cells 44, 365–393 (2014). https://doi.org/10.1146/ANNUREV-MATSCI-070813-113426
L. Malavasi, C.A.J. Fisher, M.S. Islam, Oxide-ion and proton conducting electrolyte materials for clean energy applications: structural and mechanistic features. Chem. Soc. Rev. 39, 4370–4387 (2010). https://doi.org/10.1039/B915141A
A. Tarancón, Strategies for lowering solid oxide fuel cells operating temperature. Energies 2, 1130–1150 (2009). https://doi.org/10.3390/EN20401130
M. Guzik, M. Bieza, E. Tomaszewicz, Y. Guyot, E. Zych, G. Boulon, Nd3+ dopant influence on the structural and spectroscopic properties of microcrystalline La2Mo2O9 molybdate. Opt. Mater. (Amst) 41, 21–31 (2015). https://doi.org/10.1016/J.OPTMAT.2014.11.013
T. Matsumoto, K. Sunada, T. Nagai, T. Isobe, S. Matsushita, H. Ishiguro, A. Nakajima, Preparation of hydrophobic La2Mo2O9 ceramics with antibacterial and antiviral properties. J. Hazard. Mater. (2019). https://doi.org/10.1016/j.jhazmat.2019.05.003
S. Hussain, X. Yang, M.K. Aslam, A. Shaheen, M.S. Javed, N. Aslam, B. Aslam, G. Liu, G. Qiao, R. TiN, Nanoparticles polysulfide anchor for Li–S storage and diffusion pathways using first principle calculations. Chem. Eng. J. 391, 123595 (2020). https://doi.org/10.1016/J.CEJ.2019.123595
T. Shi, H. Hou, S. Hussain, C. Ge, M.A. Alsaiari, A.S. Alkorbi, G. Liu, R. Alsaiari, G. Qiao, Efficient detection of hazardous H2S gas using multifaceted Co3O4/ZnO hollow nanostructures. Chemosphere 287, 132178 (2022). https://doi.org/10.1016/J.CHEMOSPHERE.2021.132178
A.S. Alkorbi, H.M.A. Javed, S. Hussain, S. Latif, M.S. Mahr, M.S. Mustafa, R. Alsaiari, N.A. Alhemiary, Solar light driven photocatalytic degradation of methyl blue by carbon-doped TiO2 nanoparticles. Opt. Mater. 127, 112259 (2022). https://doi.org/10.1016/J.OPTMAT.2022.112259
M. Zhan, C. Ge, S. Hussain, A.S. Alkorbi, R. Alsaiari, N.A. Alhemiary, G. Qiao, G. Liu, Enhanced NO2 gas-sensing performance by core-shell SnO2/ZIF-8 nanospheres. Chemosphere 291, 132842 (2022). https://doi.org/10.1016/J.CHEMOSPHERE.2021.132842
S. Hussain, M.S. Javed, S. Asim, A. Shaheen, A.J. Khan, Y. Abbas, N. Ullah, A. Iqbal, M. Wang, G. Qiao, S. Yun, Novel gravel-like NiMoO4 nanoparticles on carbon cloth for outstanding supercapacitor applications. Ceram. Int. 46, 6406–6412 (2020). https://doi.org/10.1016/J.CERAMINT.2019.11.118
S. Hussain, N. Farooq, A.S. Alkorbi, R. Alsaiari, N.A. Alhemiary, M. Wang, G. Qiao, Polyhedral Co3O4@ZnO nanostructures as proficient photocatalysts for vitiation of organic dyes from waste water. J. Mol. Liq. 362, 119765 (2022). https://doi.org/10.1016/J.MOLLIQ.2022.119765
J. Rodríguez-Carvajal, Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter. 192, 55–69 (1993). https://doi.org/10.1016/0921-4526(93)90108-I
M.W. Nathans, W.W. Wendlandt, The thermal decomposition of the rare-earth sulphates: thermogravimetric and differential thermal analysis studies up to 1400°C. J. Inorg. Nucl. Chem. 24, 869–879 (1962). https://doi.org/10.1016/0022-1902(62)80108-0
T.Y. Jin, M.V. Madhava Rao, C.L. Cheng, D.S. Tsai, M.H. Hung, Structural stability and ion conductivity of the Dy and W substituted La2Mo2O9. Solid State Ionics 178, 367–374 (2007). https://doi.org/10.1016/j.ssi.2007.01.031
L. Ge, K. Guo, L. Guo, Sinterability, reducibility, and electrical conductivity of fast oxide-ion conductors La1.8R0.2MoWO9 (R = Pr, Nd, Gd and Y). Ceram Int 41, 10208–11021 (2015). https://doi.org/10.1016/j.ceramint.2015.04.127
J.P. Fournier, J. Fournier, R. Kohlmuller, Bull- Google Scholar, (2022). https://scholar.google.fr/scholar?hl=en&as_sdt=0%2C5&q=J.P.+Fournier%2C+J.+Fournier%2C+R.+Kohlmuller%2C+Bull.+Soc.+J.+Chim.+Fr.+12+%281970%29+4277.&btnG (Accessed 4th Oct, 2022).
I.R. Evans, J.A.K. Howard, J.S.O. Evans, The Crystal Structure of Alpha-La2Mo2O9 and the Structural Origin of the Oxide Ion Migration Pathway. Chem. Mater. 17(16), 4074–4077 (2005). https://doi.org/10.1021/CM050049.
P. Lacorre, A. Selmi, G. Corbel, B. Boulard, On the flexibility of the structural framework of cubic LAMOX compounds, in relationship with their anionic conduction properties. Inorg. Chem. 45, 627–635 (2006). https://doi.org/10.1021/IC0513080
C. Tealdi, L. Malavasi, C. Ritter, G. Flor, G. Costa, Lattice effects in cubic La2Mo2O9: effect of vacuum and correlation with transport properties. J. Solid State Chem. 181, 603–610 (2008). https://doi.org/10.1016/J.JSSC.2008.01.001
G. Corbel, E. Suard, P. Lacorre, Structural key of the thermal expansion and the oxide ionic conduction in derivatives of La2Mo2O9: A temperature- controlled neutron diffraction study of β-La1.7Bi0.3Mo2O9. Chem. Mater. 23, 1288–1298 (2011). https://doi.org/10.1021/CM103255R/SUPPL_FILE/CM103255R_SI_002.CIF
T. Paul, A. Ghosh, Conduction and relaxation mechanisms in bismuth doped La2Mo2O9 ionic conductors. J. Appl. Phys. 114, 164101 (2013). https://doi.org/10.1063/1.4826077
S.J. Skinner, H.-D.W. Cheminform, The LAMOX family of fast oxide-ion conductors: overview and recent results. J. New Mater. Electrochem. Syst. 7, 51–57 (2004)
F. Goutenoire, O. Isnard, E. Suard, O. Bohnke, Y. Laligant, R. Retoux, Ph. Lacorre, Structural and transport characteristics of the LAMOX family of fast oxide- ion conductors, based on lanthanum molybdenum oxide La2Mo2O9. J. Mater. Chem. 11, 119–124 (2001). https://doi.org/10.1039/B002962I
B. Deb, A. Ghosh, Structure and dielectric constant of silver molybdophosphate mixed network former glasses. J. Alloys Compd. 509, 8251–8255 (2011). https://doi.org/10.1016/J.JALLCOM.2011.05.098
S.M. Abo-Naf, FTIR and UV–VIS optical absorption spectra of gamma-irradiated MoO3-doped lead borate glasses. J. Non. Cryst. Solids. 358, 406–413 (2012). https://doi.org/10.1016/J.JNONCRYSOL.2011.10.013
G. Corbel, P. Durand, P. Lacorre, Comprehensive survey of Nd3+ substitution In La2Mo2O9 oxide-ion conductor. J. Solid State Chem. 182, 1009–1016 (2009). https://doi.org/10.1016/J.JSSC.2009.01.016
M. Guzik, J. Cybińska, E. Tomaszewicz, Y. Guyot, J. Legendziewicz, G. Boulon, W. Strk, Spectroscopic behavior of Nd3+ in a new microcrystalline ZnY4W3O16 tungstate. Opt. Mater. (Amst) 34, 487–495 (2011). https://doi.org/10.1016/J.OPTMAT.2011.07.030
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) for funding and supporting this work through Research Partnership Program no RP-21-09-71.
Funding
Imam Mohammed Ibn Saud Islamic University, RP-21-09-71, Naoufel Ben Hamadi
Author information
Authors and Affiliations
Contributions
WJ contributed to formal analysis and writing–original draft; NM contributed to writing, investigation, and methodology; NBH contributed to investigation and software; AG contributed to conceptualization and validation; TS contributed to formal analysis and writing; HN contributed to methodology, validation, writing–review & editing, and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest among themselves.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jabeur, W., Mhadhbi, N., Hamadi, N.B. et al. Effect of humidified atmosphere on the physicochemical properties of S-doped La2Mo2O9 oxide-ion conductors. J Mater Sci: Mater Electron 34, 27 (2023). https://doi.org/10.1007/s10854-022-09461-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10854-022-09461-6