Abstract
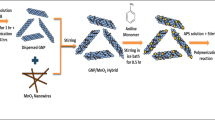
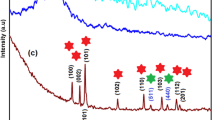
Energy shortage imposes major global issues, and developing new energy storage devices has become extremely urgent. Because supercapacitors (SCs) have been regarded as inspirational energy storage technologies. Herein, a novel rGO/PANI/MnO2 material was developed via a chemical reduction method for high-rate supercapacitors. The morphological structural & textural properties of fabricated nanostructure been investigated with X-ray diffraction, scanning electron microscope (SEM) & Brunauer–Emmett–Teller (BET). Electrochemical characterization of advanced nanocomposite contains specific capacitance (Csp) of 1613.7 F g−1 with enhanced rate capabilities and cycling stability retention of 99.7% with original capacity after 2000 cycles in 2 M KOH. Excellent efficiency of fabricated material is attributed due to small crystallite size, good morphology, and enhanced electrochemical surface area. This study emphasizes the good electrochemical performance of rGO/PANI/MnO2 on carbon fiber. The flake like morphology of the composite provides numerous electronic conduction routes, and more active sites refer it to be potential candidate for energy storage devices.








Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
B.G. Pollet, I. Staffell, J.L. Shang, V. Molkov, Fuel-cell (hydrogen) electric hybrid vehicles. Altern. Fuels Adv. Veh. Technol. Improv. Environ. Perform. Towar. Zero Carbon Transp. (2014). https://doi.org/10.1533/9780857097422.3.685
Y. Li, Y. Li, P. Ji, J. Yang, development of energy storage industry in China: a technical and economic point of review. Renew. Sustain. Energy Rev. 49, 805–812 (2015). https://doi.org/10.1016/J.RSER.2015.04.160
H. Wang, D. Tran, J. Qian et al., MoS2/graphene composites as promising materials for energy storage and conversion applications. Adv. Mater. Interfaces 6, 1900915 (2019). https://doi.org/10.1002/ADMI.201900915
H. Chen, T.N. Cong, W. Yang et al., Progress in electrical energy storage system: a critical review. Prog. Nat. Sci. 19, 291–312 (2009). https://doi.org/10.1016/J.PNSC.2008.07.014
J. Zhu, D. Yang, Z. Yin et al., Graphene and graphene-based materials for energy storage applications. Small 10, 3480–3498 (2014). https://doi.org/10.1002/SMLL.201303202
X. Wang, D. Wu, X. Song et al., Review on carbon, polyaniline hybrids: design and synthesis for supercapacitor. Page 2263(24), 2263 (2019). https://doi.org/10.3390/MOLECULES24122263
Liao CH, Huang CW, Wu JCS (2012) Hydrogen Production from Semiconductor-based Photocatalysis via Water Splitting. Catal 2012, Vol 2, Pages 490–516 2:490–516. https://doi.org/10.3390/CATAL2040490
A. Mishra, A. Mehta, S. Basu et al., Graphitic carbon nitride (g–C3N4)–based metal-free photocatalysts for water splitting: a review. Carbon N Y 149, 693–721 (2019). https://doi.org/10.1016/J.CARBON.2019.04.104
T. Ma, H. Yang, L. Lu, Development of hybrid battery–supercapacitor energy storage for remote area renewable energy systems. Appl. Energy 153, 56–62 (2015). https://doi.org/10.1016/J.APENERGY.2014.12.008
Hu X, Tseng KJ, Srinivasan M (2011) Optimization of battery energy storage system with super-capacitor for renewable energy applications. 8th Int Conf Power Electron - ECCE Asia "Green World with Power Electron ICPE 2011-ECCE Asia. 1552–1557 https://doi.org/10.1109/ICPE.2011.5944515
A. Pramitha, Y. Raviprakash, Recent developments and viable approaches for high-performance supercapacitors using transition metal-based electrode materials. J. Energy Storage 49, 104120 (2022). https://doi.org/10.1016/J.EST.2022.104120
Electrodeposition of Nickel Cobalt Oxide for the Application of Asymmetric Flexible Microsupercapacitor. http://dr.iiserpune.ac.in:8080/xmlui/handle/123456789/818. Accessed 27 May 2022
F. Qi, X. Lu, Y. Wang et al., Fabrication of hierarchical MoO3@NixCo2x(OH)6x core–shell arrays on carbon cloth as enhanced-performance electrodes for asymmetric supercapacitors. J. Colloid. Interface Sci. 607, 1253–1261 (2022). https://doi.org/10.1016/J.JCIS.2021.09.046
F. Qi, L. Shao, X. Shi et al., “Carbon quantum dots-glue” enabled high-capacitance and highly stable nickel sulphide nanosheet electrode for supercapacitors. J. Colloid. Interface Sci. 601, 669–677 (2021). https://doi.org/10.1016/J.JCIS.2021.05.099
S. Wang, L. Shao, L. Yu et al., Niobium carbide as a promising pseudocapacitive sodium-ion storage anode. Energy Technol. 9, 2100298 (2021). https://doi.org/10.1002/ENTE.202100298
Y. Pi, W. Sun, M. Yan et al., Achieving high-performance energy storage device of Li3V2(PO4)3 // LiCrTiO4 Li-ion full cell. J. Power Sources 518, 230770 (2022). https://doi.org/10.1016/J.JPOWSOUR.2021.230770
L. Pu, J. Zhang, N.K.L. Jiresse et al., N-doped MXene derived from chitosan for the highly effective electrochemical properties as supercapacitor. Adv. Compos. Hybrid. Mater. 5, 356–369 (2022). https://doi.org/10.1007/S42114-021-00371-5/FIGURES/10
M. Sivakumar, B. Muthukutty, G. Panomsuwan et al., Facile synthesis of NiFe2O4 nanoparticle with carbon nanotube composite electrodes for high-performance asymmetric supercapacitor. Colloids. Surfaces A Physicochem. Eng. Asp 648, 129188 (2022). https://doi.org/10.1016/J.COLSURFA.2022.129188
H.T. Das, T EB, Dutta S, et al., Recent trend of CeO2-based nanocomposites electrode in supercapacitor: a review on energy storage applications. J. Energy Storage 50, 104643 (2022). https://doi.org/10.1016/J.EST.2022.104643
M.S. Islam, Y. Shudo, S. Hayami, Energy conversion and storage in fuel cells and super-capacitors from chemical modifications of carbon allotropes: state-of-art and prospect. Bulletin Chem. Soc. Japan (2021). https://doi.org/10.1246/BCSJ.20210297
N. Devi, S. Sahoo, R. Kumar, R.K. Singh, A review of the microwave-assisted synthesis of carbon nanomaterials, metal oxides/hydroxides and their composites for energy storage applications. Nanoscale 13, 11679–11711 (2021). https://doi.org/10.1039/D1NR01134K
J. Luo, W. Zhong, Y. Zou et al., Preparation of morphology-controllable polyaniline and polyaniline/graphene hydrogels for high performance binder-free supercapacitor electrodes. J. Power Sources 319, 73–81 (2016). https://doi.org/10.1016/J.JPOWSOUR.2016.04.004
R.L. Razalli, M.M. Abdi, P.M. Tahir et al., Polyaniline-modified nanocellulose prepared from semantan bamboo by chemical polymerization: preparation and characterization. RSC Adv. 7, 25191–25198 (2017). https://doi.org/10.1039/C7RA03379F
T. Tao, J. He, Y. Wang et al., Microwave-assisted hydrothermal synthesis of three-dimensional NbOPO4-reduced graphene oxide-carbon nanotube composite for high performance sodium-ion battery anode. J. Power Sources 539, 231457 (2022). https://doi.org/10.1016/J.JPOWSOUR.2022.231457
S.A. Habib, S.A. Saafan, T.M. Meaz et al., Structural, magnetic, and AC measurements of nanoferrites, graphene composites. Nanomater 931(12), 931 (2022). https://doi.org/10.3390/NANO12060931
G. Liu, Y. Yang, X. Lu et al., Fully active bimetallic phosphide Zn0.5Ge0.5P: a novel high-performance anode for na-ion batteries coupled with diglyme-based electrolyte. ACS Appl. Mater. Interfaces 14, 31803–31813 (2022). https://doi.org/10.1021/ACSAMI.2C03813
R. Oraon, A. De Adhikari, S.K. Tiwari, G.C. Nayak, Enhanced specific capacitance of self-assembled three-dimensional carbon nanotube/layered silicate/polyaniline hybrid sandwiched nanocomposite for supercapacitor applications. ACS Sustain. Chem. Eng. 4, 1392–1403 (2016). https://doi.org/10.1021/ACSSUSCHEMENG.5B01389/ASSET/IMAGES/LARGE/SC-2015-013896_0014.JPEG
S. Sivakumar, L. Nelson Prabu, Synthesis and Characterization of α-MnO2 nanoparticles for Supercapacitor application. Mater. Today Proc. 47, 52–55 (2021). https://doi.org/10.1016/J.MATPR.2021.03.528
H. Fan, H. Zhang, X. Luo et al., Hydrothermal solid-gas route to TiO2 nanoparticles/nanotube arrays for high-performance supercapacitors. J. Power Sources 357, 230–240 (2017). https://doi.org/10.1016/J.JPOWSOUR.2017.05.009
S. Vijayakumar, A. Kiruthika Ponnalagi, S. Nagamuthu, G. Muralidharan, Microwave assisted synthesis of Co3O4 nanoparticles for high-performance supercapacitors. Electrochim. Acta. 106, 500–505 (2013). https://doi.org/10.1016/J.ELECTACTA.2013.05.121
T. Zahra, K.S. Ahmad, C. Zequine et al., Electro-catalyst [ZrO2/ZnO/PdO]-NPs green functionalization: fabrication, characterization and water splitting potential assessment. Int. J. Hydrogen Energy 46, 19347–19362 (2021). https://doi.org/10.1016/J.IJHYDENE.2021.03.094
L. Mohan, A.V. Avani, P. Kathirvel et al., Investigation on structural, morphological and electrochemical properties of Mn doped WO3 nanoparticles synthesized by co-precipitation method for supercapacitor applications. J. Alloys Compd. 882, 160670 (2021). https://doi.org/10.1016/J.JALLCOM.2021.160670
Y. Zhang, J. Zheng, Q. Wang et al., One-step hydrothermal preparation of (NH4)2V3O8/carbon composites and conversion to porous V2O5 nanoparticles as supercapacitor electrode with excellent pseudocapacitive capability. Appl. Surf. Sci. 423, 728–742 (2017). https://doi.org/10.1016/J.APSUSC.2017.06.249
H. Zhang, Y. Chen, Y. Wang et al., A highly electroactive poly(aniline-co-thionine) for rechargeable zinc batteries. J. Electrochem. Soc. 168, 060526 (2021). https://doi.org/10.1149/1945-7111/AC0945
C. Chen, Z. Gan, C. Xu et al., Electrosynthesis of poly(aniline-co-azure B) for aqueous rechargeable zinc-conducting polymer batteries. Electrochim. Acta. 252, 226–234 (2017). https://doi.org/10.1016/J.ELECTACTA.2017.08.195
Z. Gan, N. Song, H. Zhang et al., One-step electrofabrication of reduced graphene oxide/poly(N-methylthionine) composite film for high performance supercapacitors. J. Electrochem. Soc. 167, 085501 (2020). https://doi.org/10.1149/1945-7111/AB8C82
M. Ramezani, M. Fathi, F. Mahboubi, Facile synthesis of ternary MnO2/graphene nanosheets/carbon nanotubes composites with high rate capability for supercapacitor applications. Electrochim. Acta. 174, 345–355 (2015). https://doi.org/10.1016/J.ELECTACTA.2015.05.155
M. Kumar, X. Xiong, Z. Wan et al., Ball milling as a mechanochemical technology for fabrication of novel biochar nanomaterials. Bioresour. Technol. 312, 123613 (2020). https://doi.org/10.1016/J.BIORTECH.2020.123613
H. Takahashi, H. Fujiki, S. Yokoyama et al., Synthesis of carbon nanomaterials from different pyrolysis techniques: a review. Mater. Res. Express 5, 052002 (2018). https://doi.org/10.1088/2053-1591/AAC05B
T.A. Nguyen, V.N.T. Pham, H.T. Le et al., Crystal structure and magnetic properties of LaFe1-xNixO3 nanomaterials prepared via a simple co-precipitation method. Ceram. Int. 45, 21768–21772 (2019). https://doi.org/10.1016/J.CERAMINT.2019.07.178
K.H. Tam, C.K. Cheung, Y.H. Leung et al., Defects in ZnO nanorods prepared by a hydrothermal method. J. Phys. Chem. B 110, 20865–20871 (2006). https://doi.org/10.1021/JP063239W/ASSET/IMAGES/LARGE/JP063239WF00008.JPEG
N. Liu, X. Chen, J. Zhang, J.W. Schwank, A review on TiO2-based nanotubes synthesized via hydrothermal method: formation mechanism, structure modification, and photocatalytic applications. Catal. Today 225, 34–51 (2014). https://doi.org/10.1016/J.CATTOD.2013.10.090
L. Huang, F. Peng, H. Wang et al., Preparation and characterization of Cu2O/TiO2 nano–nano heterostructure photocatalysts. Catal. Commun. 10, 1839–1843 (2009). https://doi.org/10.1016/J.CATCOM.2009.06.011
M.M. Aljumaily, M.A. Alsaadi, R. Das et al., Optimization of the synthesis of superhydrophobic carbon nanomaterials by chemical vapor deposition. Sci. Reports 81(8), 1–12 (2018). https://doi.org/10.1038/s41598-018-21051-3
S. Subhadarshini, R. Singh, A. Mandal et al., Silver nanodot decorated dendritic copper foam as a hydrophobic and mechano-chemo bactericidal surface. Langmuir 37, 9356–9370 (2021). https://doi.org/10.1021/ACS.LANGMUIR.1C00698/ASSET/IMAGES/LARGE/LA1C00698_0008.JPEG
D. Zhao, M. Dai, H. Liu et al., Sulfur-induced interface engineering of hybrid NiCo2O4@NiMo2S4 structure for overall water splitting and flexible hybrid energy storage. Adv. Mater. Interfaces 6, 1901308 (2019). https://doi.org/10.1002/ADMI.201901308
R.B. Pujari, A.C. Lokhande, J.H. Kim, C.D. Lokhande, Bath temperature controlled phase stability of hierarchical nanoflakes CoS2 thin films for supercapacitor application. RSC Adv. 6, 40593–40601 (2016). https://doi.org/10.1039/C6RA06442F
J. Yu, N. Fu, J. Zhao et al., High specific capacitance electrode material for supercapacitors based on resin-derived nitrogen-doped porous carbons. ACS Omega 4, 15904–15911 (2019). https://doi.org/10.1021/ACSOMEGA.9B01916/ASSET/IMAGES/MEDIUM/AO9B01916_M004.GIF
W.Q. Chen, J. Wang, K.Y. Ma et al., Hierarchical NiCo2O4@Co-Fe LDH core-shell nanowire arrays for high-performance supercapacitor. Appl. Surf. Sci. 451, 280–288 (2018). https://doi.org/10.1016/J.APSUSC.2018.04.254
D.P. Dubal, R. Holze, All-solid-state flexible thin film supercapacitor based on Mn3O4 stacked nanosheets with gel electrolyte. Energy 51, 407–412 (2013). https://doi.org/10.1016/J.ENERGY.2012.11.021
K. Song, X. Wang, J. Wang et al., Bioinspired reduced graphene oxide/polyacrylonitrile-based carbon fibers/CoFe2O4 nanocomposite for flexible supercapacitors with high strength and capacitance. ChemElectroChem 5, 1297–1305 (2018). https://doi.org/10.1002/CELC.201800004
H.S. Tripathi, A. Dutta, T.P. Sinha, Tailoring structural and electrochemical properties in Sr2+ incorporated nanostructured BiFeO3 for enhanced asymmetric solidstate supercapacitor. Electrochim. Acta 421, 140505 (2022). https://doi.org/10.1016/J.ELECTACTA.2022.140505
Q. Zhang, Z. Liu, B. Zhao et al., Design and understanding of dendritic mixed-metal hydroxide nanosheets@N-doped carbon nanotube array electrode for high-performance asymmetric supercapacitors. Energy Storage Mater. 16, 632–645 (2019). https://doi.org/10.1016/J.ENSM.2018.06.026
M. Kim, Y.K. Kim, J. Kim et al., Fabrication of a polyaniline/MoS2 nanocomposite using self-stabilized dispersion polymerization for supercapacitors with high energy density. RSC Adv. 6, 27460–27465 (2016). https://doi.org/10.1039/C6RA00797J
J. Manuel, T. Salguero, R.P. Ramasamy, Synthesis and characterization of polyaniline nanofibers as cathode active material for sodium-ion battery. J. Appl. Electrochem. 49, 529–537 (2019). https://doi.org/10.1007/S10800-019-01298-Y/FIGURES/10
T. Munawar, M.N. Rehman, ur, Nadeem MS, et al., Facile synthesis of Cr-Co co-doped CdO nanowires for photocatalytic, antimicrobial, and supercapacitor applications. J. Alloys Compd. 885, 160885 (2021). https://doi.org/10.1016/J.JALLCOM.2021.160885
S. Moopri Singer Pandiyarajan, G.K. Veerasubramani, R.M. Bhattarai et al., Designing an interlayer-widened MoS2-Packed nitrogen-rich carbon nanotube core-shell structure for redox-mediated quasi-solid-state supercapacitors. ACS Appl. Energy Mater. 4, 2218–2230 (2021). https://doi.org/10.1021/ACSAEM.0C02739/ASSET/IMAGES/LARGE/AE0C02739_0009.JPEG
J.H. Kim, K.H. Lee, L.J. Overzet, G.S. Lee, Synthesis and electrochemical properties of spin-capable carbon nanotube sheet/MnOx composites for high-performance energy storage devices. Nano Lett. 11, 2611–2617 (2011). https://doi.org/10.1021/NL200513A/SUPPL_FILE/NL200513A_SI_001.PDF
M. Dirican, M. Yanilmaz, A.M. Asiri, X. Zhang, Polyaniline/MnO2/porous carbon nanofiber electrodes for supercapacitors. J. Electroanal. Chem. 861, 113995 (2020). https://doi.org/10.1016/J.JELECHEM.2020.113995
J.R.I. Jaidev, A.K. Mishra, S. Ramaprabhu, Polyaniline–MnO2 nanotube hybrid nanocomposite as supercapacitor electrode material in acidic electrolyte. J Mater Chem 21, 17601–17605 (2011). https://doi.org/10.1039/C1JM13191E
F. Meng, X. Yan, Y. Zhu, P. Si, Controllable synthesis of MnO2/polyaniline nanocomposite and its electrochemical capacitive property. Nanoscale Res Lett 8, 1–8 (2013). https://doi.org/10.1186/1556-276X-8-179/FIGURES/6
M.W. Ahmad, S. Anand, A. Fatima et al., Facile synthesis of copper oxide nanoparticles-decorated polyaniline nanofibers with enhanced electrochemical performance as supercapacitor electrode. Polym. Adv. Technol. 32, 4070–4081 (2021). https://doi.org/10.1002/PAT.5414
S. Rai, R. Bhujel, J. Biswas, B.P. Swain, An eco-friendly method for synthesis of Cu2O/rGO/PANI composite using citrus maxima juice for supercapacitor application. J. Mater. Sci. Mater. Electron. 32, 27937–27949 (2021). https://doi.org/10.1007/S10854-021-07175-9/TABLES/2
S. Palsaniya, H.B. Nemade, A.K. Dasmahapatra, Hierarchical PANI-RGO-ZnO ternary nanocomposites for symmetric tandem supercapacitor. J. Phys. Chem. Solids 154, 110081 (2021). https://doi.org/10.1016/J.JPCS.2021.110081
B. Purty, R.B. Choudhary, R. Kandulna, R. Singh, Remarkable enhancement in electrochemical capacitance value of Ag-ZnO/PANI composite for supercapacitor application. AIP Conf. Proc. 2115, 030588 (2019). https://doi.org/10.1063/1.5113427
R. Karthik, S. Thambidurai, Synthesis of RGO–Co doped ZnO/PANI hybrid composite for supercapacitor application. J. Mate. Sci. Mater. Electron. 28, 9836–9851 (2017). https://doi.org/10.1007/S10854-017-6738-4/FIGURES/10
B P P, D N A, M S R, Kumar K Y, Synthesis of polyaniline/α-Fe2O3 nanocomposite electrode material for supercapacitor applications. Mater. Today Commun. 12, 72–78 (2017). https://doi.org/10.1016/J.MTCOMM.2017.07.002
Z. Qiu, Y. Peng, D. He et al., Ternary Fe3O4@C@PANi nanocomposites as high-performance supercapacitor electrode materials. J. Mater. Sci. 53, 12322–12333 (2018). https://doi.org/10.1007/S10853-018-2451-9/FIGURES/7
X. Liu, J. Wang, G. Yang, In situ growth of the Ni3V2O8@PANI composite electrode for flexible and transparent symmetric supercapacitors. ACS Appl. Mater. Interfaces 10, 20688–20695 (2018). https://doi.org/10.1021/ACSAMI.8B04609/ASSET/IMAGES/LARGE/AM-2018-04609R_0006.JPEG
Naveed ur Rehman M, Munawar T, Nadeem MS, et al., Facile synthesis and characterization of conducting polymer-metal oxide based core-shell PANI-Pr2O–NiO–Co3O4 nanocomposite: as electrode material for supercapacitor. Ceram. Int. 47, 18497–18509 (2021). https://doi.org/10.1016/J.CERAMINT.2021.03.173
S. Phokha, S. Hunpratub, N. Chanlek et al., Synthesis, characterization and electrochemical performance of carbon/Ni-doped CeO2 composites. J. Alloys Compd. 750, 788–797 (2018). https://doi.org/10.1016/J.JALLCOM.2018.04.053
Acknowledgements
The authors are very thankful to Bahauddin Zakariya University, Multan, for financial support under project number DR & EL/D-545. The authors would like to acknowledge the financial support of Taif University Researchers Supporting Project number (TURSP-2020/189), Taif University, Taif, Saudi Arabia.
Funding
This study was funded by Bahauddin Zakariya University,R&D545, Muhammad Naeem Ashiq, Taif University, TURSP-2020/189, K. H. Mahmoud.
Author information
Authors and Affiliations
Contributions
All have done equal contribution.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article has compliance with ethical standards of journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abbas, S., Manzoor, S., Abdullah, M. et al. One-pot synthesis of reduced graphene oxide-based PANI/MnO2 ternary nanostructure for high-efficiency supercapacitor applications. J Mater Sci: Mater Electron 33, 25355–25370 (2022). https://doi.org/10.1007/s10854-022-09242-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-09242-1